
The structure of diborane contains.
A. four \[2c - 2e\] bonds and two \[3c - 2e\] bonds
B two \[2c - 2e\] bonds and four \[3c - 2e\] bonds
C. two \[2c - 2e\] bonds and two \[3c - 2e\] bonds
D. four \[2c - 2e\] bonds and four \[3c - 2e\] bonds
Answer
578.1k+ views
Hint: Boron is \[s{p^3}\] hybridized, so the total number of bonds formed by each boron atom is four. Also consider the total number of electrons involved in bonding will determine the type of bonding.
Complete step by step answer:
Boron is an element in the periodic table with atomic number \[5\]. The electronic configuration of boron is:
\[B\]: \[1{s^2}2{s^2}2{p^1}\] (in ground state)
\[B\]: \[1{s^2}2{s^1}2p{x^1}2p{y^1}\] (in excited state).
The valence shell configuration of boron orbitals can be shown as:
\[B\] in ground state:
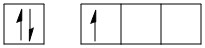
\[B\] in hybridized state:
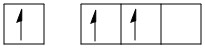
The bonding of the boron in \[{B_2}{H_6}\] can be shown as:
\[{B_2}{H_6}\]:
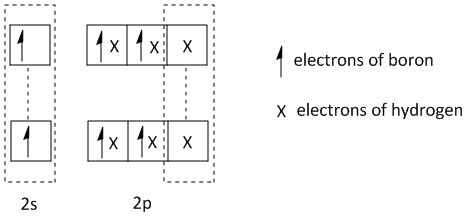
Clearly from the above structure of orbitals, two different types of bonding orbitals are present in \[{B_2}{H_6}\] molecules. The hybridization of the boron atom is \[s{p^3}\]. Four of the hybrid orbitals are forming sigma bonds directly to the hydrogen atoms. The electrons involved in this type of bond are one electron from boron and one electron from hydrogen. So it is a two centre two electron bond.
The other two hybrid orbitals form two bridging bonds between the central boron atoms. This type of bridging bond is also known as a banana bond consisting of \[B - H - B\] bonds. Here one electron each from two boron atoms and one electron from the hydrogen atom are involved. So it is a three centre two electron bond.
Thus the correct option is A, i.e. four \[2c - 2e\] bonds and two \[3c - 2e\] bonds.
Note:
In general the electron deficient bonds are bridge bonds. The number of electrons involved in such bonds is less than the normal. Boron and aluminium are known to form such compounds by sharing electrons from bonded atoms.
Complete step by step answer:
Boron is an element in the periodic table with atomic number \[5\]. The electronic configuration of boron is:
\[B\]: \[1{s^2}2{s^2}2{p^1}\] (in ground state)
\[B\]: \[1{s^2}2{s^1}2p{x^1}2p{y^1}\] (in excited state).
The valence shell configuration of boron orbitals can be shown as:
\[B\] in ground state:

\[B\] in hybridized state:

The bonding of the boron in \[{B_2}{H_6}\] can be shown as:
\[{B_2}{H_6}\]:

Clearly from the above structure of orbitals, two different types of bonding orbitals are present in \[{B_2}{H_6}\] molecules. The hybridization of the boron atom is \[s{p^3}\]. Four of the hybrid orbitals are forming sigma bonds directly to the hydrogen atoms. The electrons involved in this type of bond are one electron from boron and one electron from hydrogen. So it is a two centre two electron bond.
The other two hybrid orbitals form two bridging bonds between the central boron atoms. This type of bridging bond is also known as a banana bond consisting of \[B - H - B\] bonds. Here one electron each from two boron atoms and one electron from the hydrogen atom are involved. So it is a three centre two electron bond.
Thus the correct option is A, i.e. four \[2c - 2e\] bonds and two \[3c - 2e\] bonds.
Note:
In general the electron deficient bonds are bridge bonds. The number of electrons involved in such bonds is less than the normal. Boron and aluminium are known to form such compounds by sharing electrons from bonded atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




