
The sodium phenoxide ion formed during the preparation of phenol from benzene sulphonic hydrolysed in presence of acid to:
A.Neutralize the basic phenoxide ion
B.To increase the basic nature of the reaction mixture
C.To acidify the reaction mixture so as to get phenol
D.Both A and C
Answer
590.1k+ views
Hint:We can prepare benzene sulphonic acid from benzene. Benzene is reacted with oleum (fuming sulfuric acid) to get benzene sulphonic acid. We can react with benzene sulfonic acid with a base to form phenol.
Complete step by step answer:
We know that phenol is an aromatic organic compound that has a chemical formula of ${C_6}{H_5}OH.$ it appears as crystalline solid, white in color and is volatile.
We know that benzene is used in the preparation of benzene sulfonic acid.
We can synthesize benzene sulfonic acid by reacting benzene with oleum. Oleum is fuming sulfuric acid. We can write the chemical reaction for the synthesis of benzene sulfonic acid as,

We now have to treat the benzene sulfonic acid with an aqueous solution of sodium hydroxide to give sodium benzene sulfonate ion. We can write this reaction as,

We have to mix the resulting salt with solid sodium hydroxide and fuse them at high temperatures. The product obtained during this reaction is sodium phenoxide ion. We can write the chemical reaction as,

The sodium phenoxide ion is then acidified with aqueous acid to form phenol. We can write the chemical reaction for this step as,
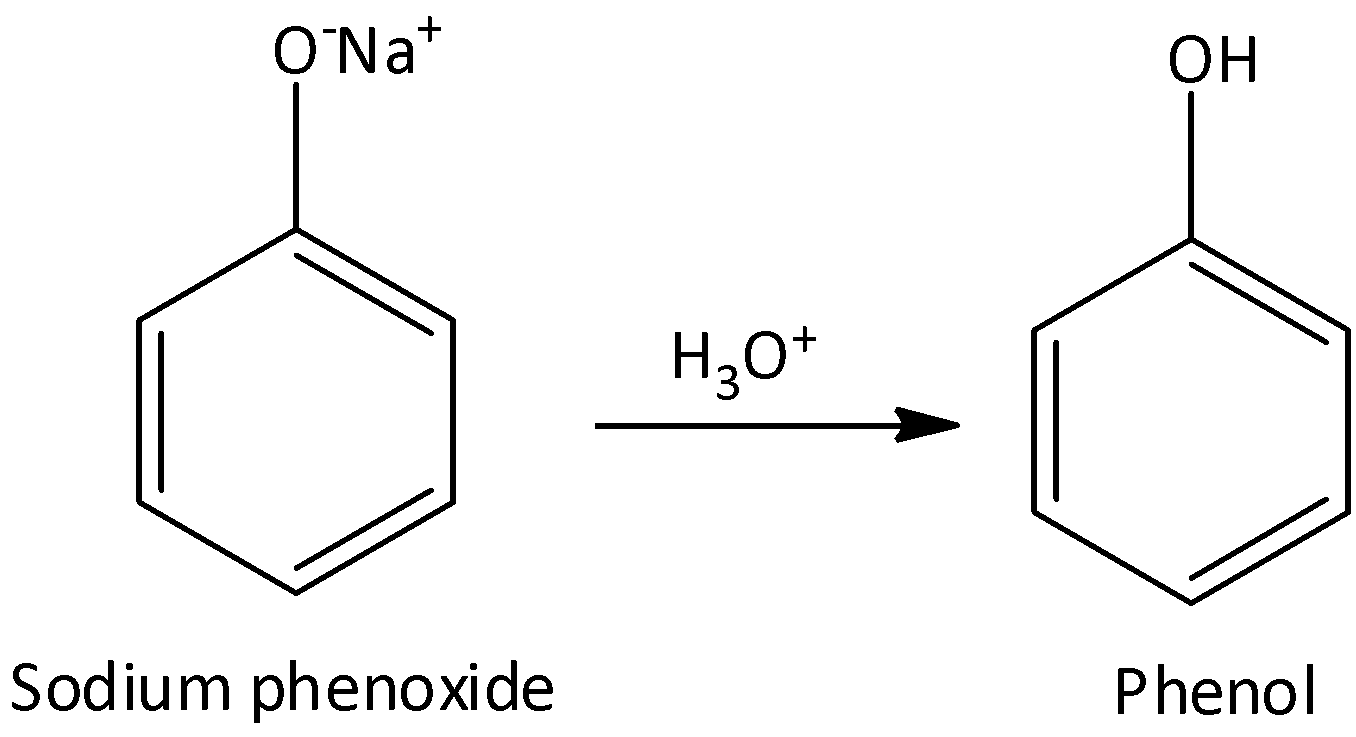
The sodium phenoxide ion formed during the preparation of phenol from benzene sulphonic hydrolysed in presence of acid to neutralize the basic phenoxide ion and acidifies the mixture to yield phenol.
$\therefore $Option (D) is correct.
Note:
We can also prepare phenols using,
Haloarenes
Diazonium salts
Cumene
Chlorination of benzene
We can use phenols in the production of plastics and materials related to plastic. It is also used in the production of polycarbonates, epoxies, detergents, Bakelite, pharmaceutical drugs etc.
Complete step by step answer:
We know that phenol is an aromatic organic compound that has a chemical formula of ${C_6}{H_5}OH.$ it appears as crystalline solid, white in color and is volatile.
We know that benzene is used in the preparation of benzene sulfonic acid.
We can synthesize benzene sulfonic acid by reacting benzene with oleum. Oleum is fuming sulfuric acid. We can write the chemical reaction for the synthesis of benzene sulfonic acid as,

We now have to treat the benzene sulfonic acid with an aqueous solution of sodium hydroxide to give sodium benzene sulfonate ion. We can write this reaction as,

We have to mix the resulting salt with solid sodium hydroxide and fuse them at high temperatures. The product obtained during this reaction is sodium phenoxide ion. We can write the chemical reaction as,

The sodium phenoxide ion is then acidified with aqueous acid to form phenol. We can write the chemical reaction for this step as,

The sodium phenoxide ion formed during the preparation of phenol from benzene sulphonic hydrolysed in presence of acid to neutralize the basic phenoxide ion and acidifies the mixture to yield phenol.
$\therefore $Option (D) is correct.
Note:
We can also prepare phenols using,
Haloarenes
Diazonium salts
Cumene
Chlorination of benzene
We can use phenols in the production of plastics and materials related to plastic. It is also used in the production of polycarbonates, epoxies, detergents, Bakelite, pharmaceutical drugs etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




