
The smell of bitter almond is given by the compound:
A) benzoic acid
B) benzaldehyde
C) vanillin
D) cinnamaldehyde
Answer
558.9k+ views
Hint:Smell is also called Aroma. The compound is smelly because of their structures. The class of the organic compounds which are smelly are called aromatic compounds.
The aromatic compounds are the compounds which are cyclic, planar, conjugated, obeys the Huckel rule of aromaticity. The aromatic compounds are stable compounds.
Complete answer:
Here, option(A) given is benzoic acid which is colourless crystalline solid which has a faint pleasantodour. The structure of benzoic acid is as follows:
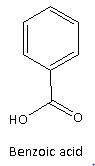
Therefore, option(A) is incorrect.
Here, option(B) given is benzaldehyde it is a colourless liquid and it has almond-like odour. The structure of the benzaldehyde is as follows:
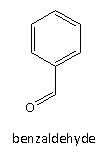
Therefore, option(B) is the correct answer to the question.
Now, option(C) given is vanillin and its structure is as follows

Vanillin is white crystalline solid and it has sweet, pleasant odour hence, option(D) is incorrect.
Here, option(D) given is cinnamaldehyde and its structure is given as follows:
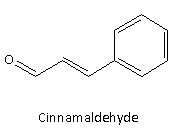
It is yellow coloured oil and it has pungent cinnamon-like odour. Therefore, option(D) is incorrect.
Hence,the correct option is (B).
Note:Here, all given compounds are the aromatic compounds.Only those compounds which are volatile can be smelled by the organism. The vapours of the compounds are diffused in the air then animals can recognise it because of the receptors present in their nose.The sensory neuron present in the nervous system possesses olfactory receptors which are useful in the recognition of smell in organisms.
The aromatic compounds are the compounds which are cyclic, planar, conjugated, obeys the Huckel rule of aromaticity. The aromatic compounds are stable compounds.
Complete answer:
Here, option(A) given is benzoic acid which is colourless crystalline solid which has a faint pleasantodour. The structure of benzoic acid is as follows:

Therefore, option(A) is incorrect.
Here, option(B) given is benzaldehyde it is a colourless liquid and it has almond-like odour. The structure of the benzaldehyde is as follows:

Therefore, option(B) is the correct answer to the question.
Now, option(C) given is vanillin and its structure is as follows

Vanillin is white crystalline solid and it has sweet, pleasant odour hence, option(D) is incorrect.
Here, option(D) given is cinnamaldehyde and its structure is given as follows:

It is yellow coloured oil and it has pungent cinnamon-like odour. Therefore, option(D) is incorrect.
Hence,the correct option is (B).
Note:Here, all given compounds are the aromatic compounds.Only those compounds which are volatile can be smelled by the organism. The vapours of the compounds are diffused in the air then animals can recognise it because of the receptors present in their nose.The sensory neuron present in the nervous system possesses olfactory receptors which are useful in the recognition of smell in organisms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




