
The product formed is:
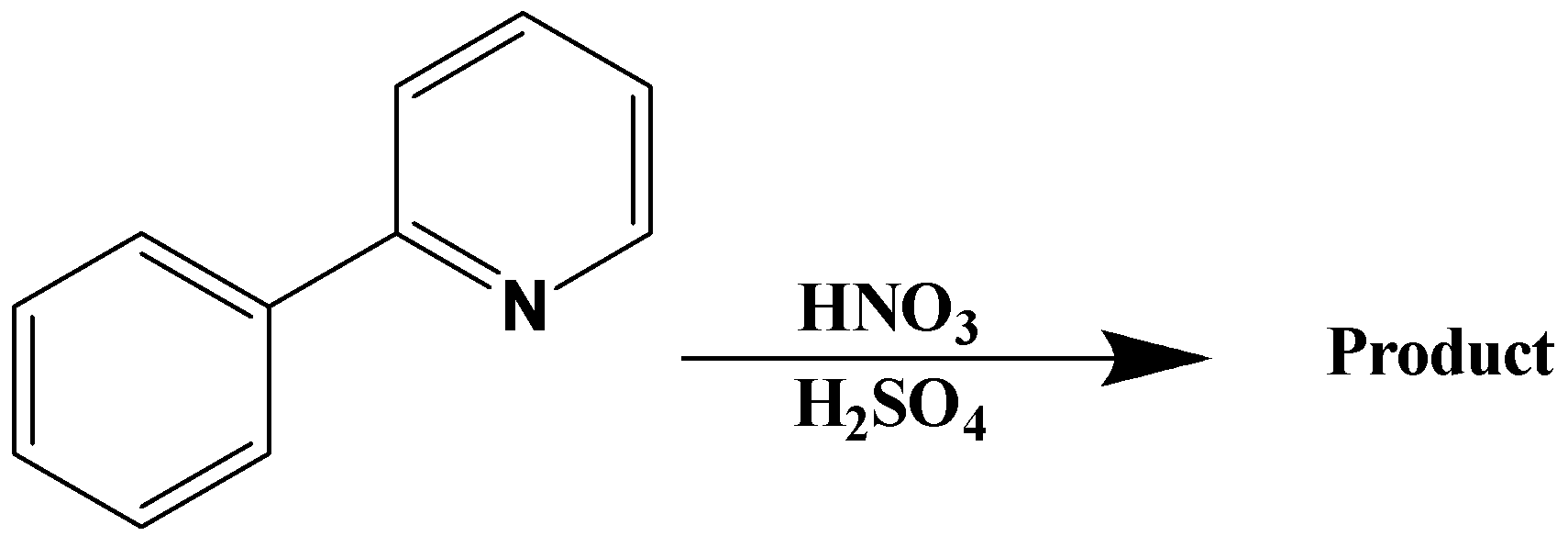
A.
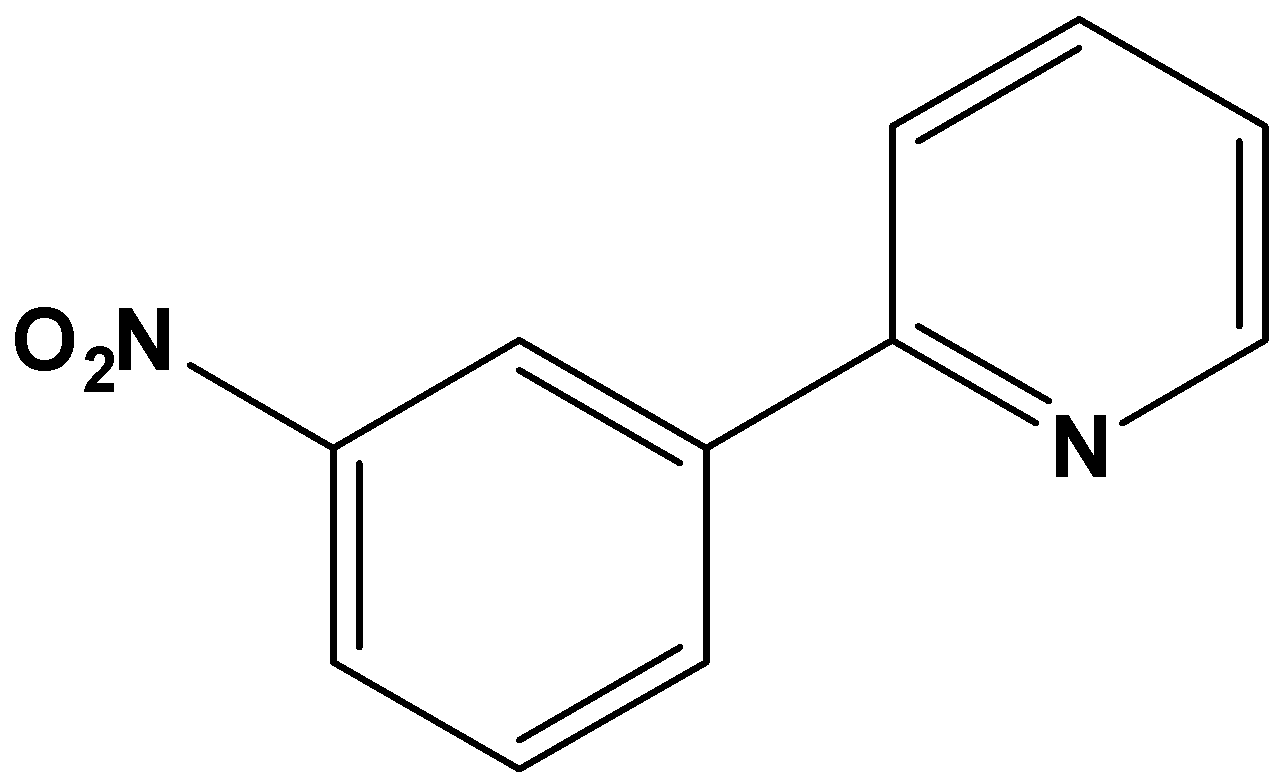
B.
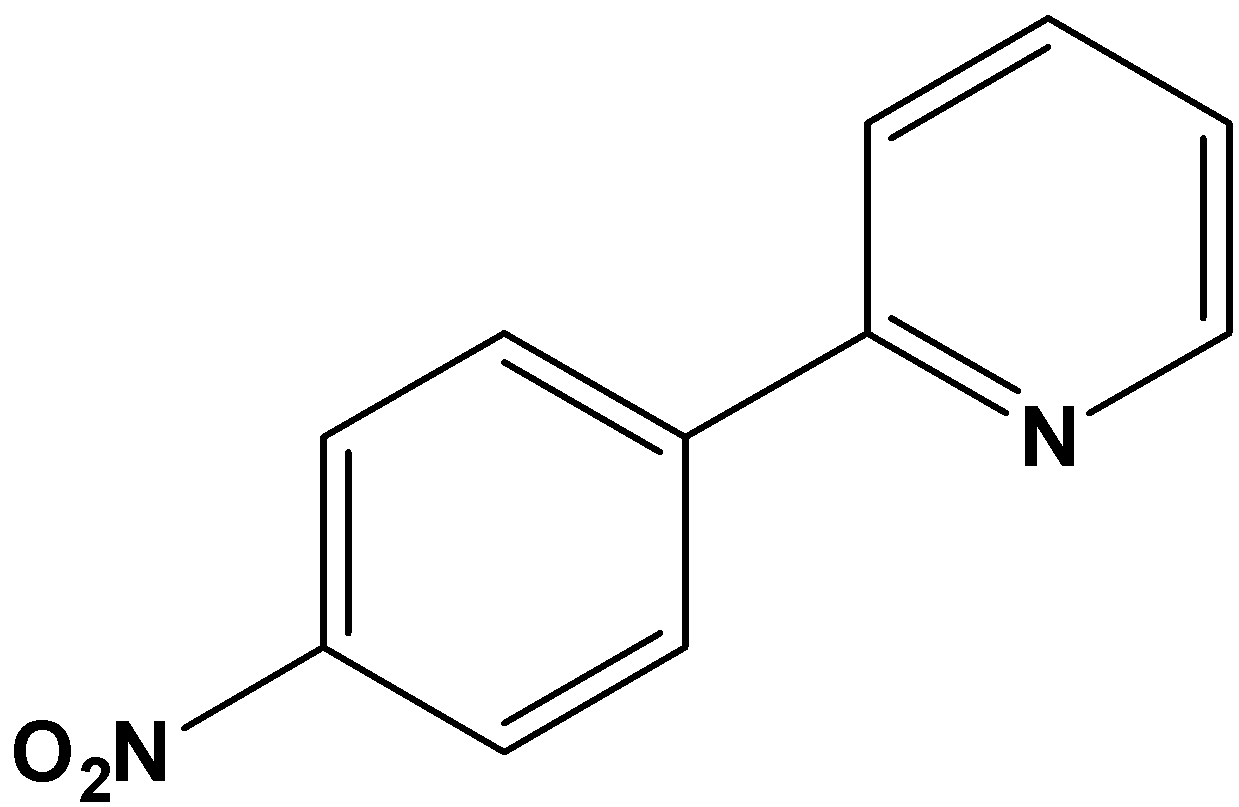
C. Pyridine acts as deactivating and o and p – directing
D. Pyridine acts as a directing and meta directing



Answer
561k+ views
Hint: The substituents in the benzene ring can be classified as activating and deactivating groups. The activating groups are electron-donating whereas deactivating groups are electron-withdrawing groups. The activating groups favour ortho and para positions in the benzene ring whereas deactivating groups favour meta-position in the benzene ring.
Complete step by step answer:
It is clear from the question that the substitution of the nitro group occurred on the benzene ring. Thus, pyridine also acts as one of the substituents in the benzene ring. Pyridine comes under the category of deactivating groups. Thus, pyridine must allow the upcoming "nitro" substituent in the benzene ring towards meta direction since pyridine is a deactivating group. The reaction obtained on nitration can be written as,
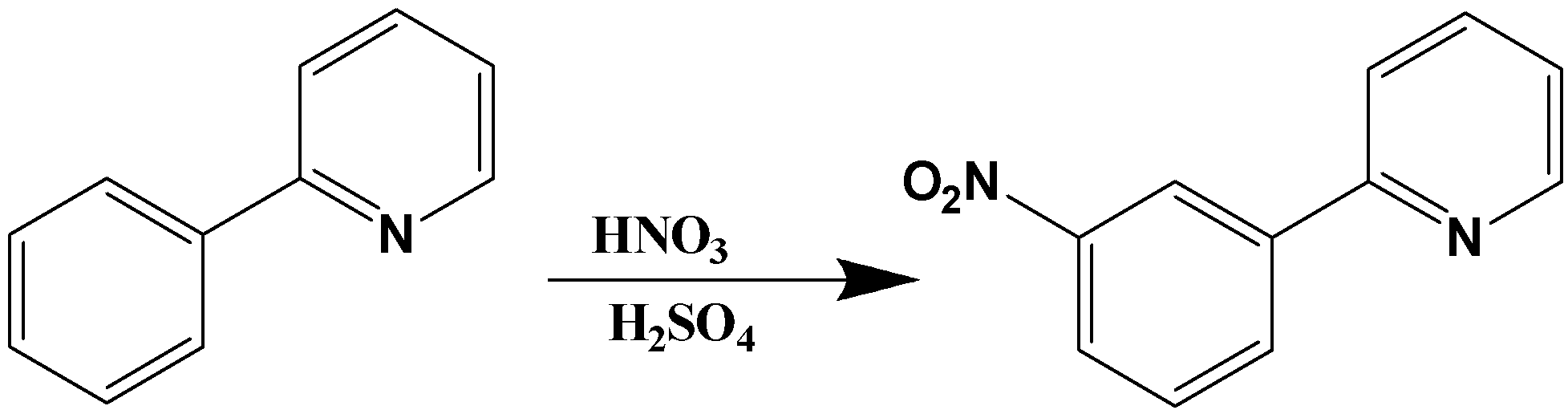
Generally, the activating group increases the rate of electrophilic substitution in the benzene ring since it increases the electron density of the benzene ring. The well-known example for activating groups is methyl (or) alkyl group, amine, alcohol group, etc... The deactivating group decreases the rate of electrophilic substitution in the benzene ring since it decreases the electron density of the benzene ring. The well-known example of deactivating groups is carbonyl group, nitro, ester, pyridine, and cyanide group, etc...
So, the correct answer is Option A.
Note: When one activating and one deactivating group are present in the benzene ring, then the upcoming substitution in the benzene ring will direct its orientation favourable to the activating group since the activating group has high electron density. The molecular formula for pyridine is \[{C_6}{H_5}N\] . It is a neutral and strong magnetic field ligand. Generally, pyridine is used as a solvent. It is used in the preparation of vitamins, drugs, and insecticides.
Complete step by step answer:
It is clear from the question that the substitution of the nitro group occurred on the benzene ring. Thus, pyridine also acts as one of the substituents in the benzene ring. Pyridine comes under the category of deactivating groups. Thus, pyridine must allow the upcoming "nitro" substituent in the benzene ring towards meta direction since pyridine is a deactivating group. The reaction obtained on nitration can be written as,

Generally, the activating group increases the rate of electrophilic substitution in the benzene ring since it increases the electron density of the benzene ring. The well-known example for activating groups is methyl (or) alkyl group, amine, alcohol group, etc... The deactivating group decreases the rate of electrophilic substitution in the benzene ring since it decreases the electron density of the benzene ring. The well-known example of deactivating groups is carbonyl group, nitro, ester, pyridine, and cyanide group, etc...
So, the correct answer is Option A.
Note: When one activating and one deactivating group are present in the benzene ring, then the upcoming substitution in the benzene ring will direct its orientation favourable to the activating group since the activating group has high electron density. The molecular formula for pyridine is \[{C_6}{H_5}N\] . It is a neutral and strong magnetic field ligand. Generally, pyridine is used as a solvent. It is used in the preparation of vitamins, drugs, and insecticides.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




