
The numbers of lone pairs and bond pairs in hydrazine are, respectively.
A.$2$ and $4$
B.$2$ and $6$
C.$2$ and $5$
D.$1$ and $5$
Answer
585.3k+ views
Hint:Hydrazine has a chemical formula ${N_2}{H_4}$. So, if we arrange two nitrogens and four hydrogens in such a way that it will be most stable. Then, we will be able to find numbers of lone pairs and bond pairs in hydrazine.
Complete step by step answer:
Hydrazine is an inorganic compound which has the chemical formula ${N_2}{H_4}$. It is colourless and flammable liquid having ammonia like odor.
Hydrazine $\left( {{N_2}{H_4}} \right)$ is one of the series of compounds called hydro nitrogens and a powerful reducing agent. It readily absorbs moisture to form the hydrate that is ${N_2}{H_4}.{H_2}O$.
Hydrazine reacts with acids and some kinds of metallic salts and the product which is formed are used in the manufacturing of certain explosives and agricultural fungicides.
Hydrazine reacts with organic compounds to form alkyl hydrazine which is used in rockets and jet propulsion.
Other products which are being formed by the reaction with organic compounds are hydrazones and hydrazides which are used in pharmaceuticals as isoniazid (used in the treatment of tuberculosis) and also used as chemical intermediates in the production of polymers and photographic chemicals.
Hydrazine is a highly reactive base and reducing agent used in many medical and industrial applications. It is also known as diamine and belongs to the class of inorganic compounds known as homogeneous other non-metal compounds.
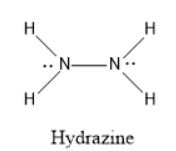
If we see the structure of hydrazine, there are two lone pairs present (one lone pair present on each nitrogen) and have five bond pairs( one between the two nitrogen atoms and four bonds present between nitrogen and hydrogen atoms.
Therefore, Option C is correct.
The numbers of lone pairs and bond pairs in hydrazine are $2$ and $5$ respectively.
Note:
Hydrazine has been found in human liver and kidney tissues and it is also detected in multiple biofluids, such as urine and blood.
In the body, hydrazine is located in the cytoplasm. It is formally rated as a possible carcinogen and also a potentially toxic compound.
Complete step by step answer:
Hydrazine is an inorganic compound which has the chemical formula ${N_2}{H_4}$. It is colourless and flammable liquid having ammonia like odor.
Hydrazine $\left( {{N_2}{H_4}} \right)$ is one of the series of compounds called hydro nitrogens and a powerful reducing agent. It readily absorbs moisture to form the hydrate that is ${N_2}{H_4}.{H_2}O$.
Hydrazine reacts with acids and some kinds of metallic salts and the product which is formed are used in the manufacturing of certain explosives and agricultural fungicides.
Hydrazine reacts with organic compounds to form alkyl hydrazine which is used in rockets and jet propulsion.
Other products which are being formed by the reaction with organic compounds are hydrazones and hydrazides which are used in pharmaceuticals as isoniazid (used in the treatment of tuberculosis) and also used as chemical intermediates in the production of polymers and photographic chemicals.
Hydrazine is a highly reactive base and reducing agent used in many medical and industrial applications. It is also known as diamine and belongs to the class of inorganic compounds known as homogeneous other non-metal compounds.

If we see the structure of hydrazine, there are two lone pairs present (one lone pair present on each nitrogen) and have five bond pairs( one between the two nitrogen atoms and four bonds present between nitrogen and hydrogen atoms.
Therefore, Option C is correct.
The numbers of lone pairs and bond pairs in hydrazine are $2$ and $5$ respectively.
Note:
Hydrazine has been found in human liver and kidney tissues and it is also detected in multiple biofluids, such as urine and blood.
In the body, hydrazine is located in the cytoplasm. It is formally rated as a possible carcinogen and also a potentially toxic compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




