
The number of structurally isomeric esters with molecular formula \[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\] are
A) 3
B) 5
C) 6
D) 8
Answer
563.4k+ views
Hint: Using the given molecular formula of ester determines the degrees of unsaturation. Ester is a carboxylic derivative of acid. The general formula of the ester is \[{\text{RCOOR'}}\]. Isomers are the compound having the same molecular formula but a different structural arrangement.
Complete solution:
We have to determine the structural isomers of ester having molecular formula\[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\].
To determine the structures of isomers we have to determine the index of hydrogen deficiency (IHD) which is also known as units of unsaturation using the following formula.
\[{\text{IHD = 0}}{\text{.5}} \times {\text{[2c + 2 - h - x + n]}}\]
Where,
c = number of carbon atoms = 5
h = number of hydrogen atoms = 10
x = number of halogen atoms = 0
n = number of nitrogen atoms = 0
\[{\text{IHD = 0}}{\text{.5}} \times {\text{[2}} \times {\text{5 + 2 - 10 - 0 + 0]}}\]
\[{\text{IHD = }}\]1
This indicates that ester having molecular formula\[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\] contains one double bond of carbonyl carbon.
The general structure of ester is
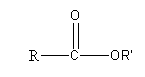
Here, R and R’ are two different alkyl groups. By using a different type of alkyl groups we can draw the different isomers of ester having a formula\[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\] as follows:
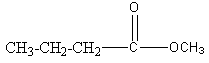
Structure I
Now we will keep \[{\text{COOC}}{{\text{H}}_{\text{3}}}\] the group fix and will write the other possible isomers.
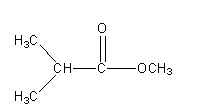
Structure II
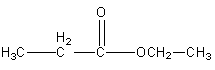
Now we will write the isomers having a different OR’ group.
Structure III
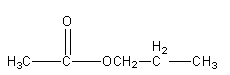
Structure IV
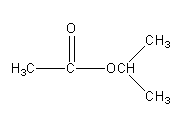
So, there are 5 isomeric esters possible having molecular formula \[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\] .
Hence, the option (B) is the correct answer to the question.
Note: Ester is one of the organic functional groups. The molecular formula of all isomers is the same only the arrangement of bonding atoms is different. Ester contains two types of alkyl groups. One alkyl group bonded to carbonyl carbon and one alkyl bonded to the oxygen atom of the carboxylate group. By varying the alkyl groups we can draw all possible isomers of ester.
Complete solution:
We have to determine the structural isomers of ester having molecular formula\[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\].
To determine the structures of isomers we have to determine the index of hydrogen deficiency (IHD) which is also known as units of unsaturation using the following formula.
\[{\text{IHD = 0}}{\text{.5}} \times {\text{[2c + 2 - h - x + n]}}\]
Where,
c = number of carbon atoms = 5
h = number of hydrogen atoms = 10
x = number of halogen atoms = 0
n = number of nitrogen atoms = 0
\[{\text{IHD = 0}}{\text{.5}} \times {\text{[2}} \times {\text{5 + 2 - 10 - 0 + 0]}}\]
\[{\text{IHD = }}\]1
This indicates that ester having molecular formula\[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\] contains one double bond of carbonyl carbon.
The general structure of ester is

Here, R and R’ are two different alkyl groups. By using a different type of alkyl groups we can draw the different isomers of ester having a formula\[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\] as follows:
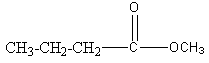
Structure I
Now we will keep \[{\text{COOC}}{{\text{H}}_{\text{3}}}\] the group fix and will write the other possible isomers.

Structure II

Now we will write the isomers having a different OR’ group.
Structure III
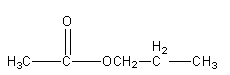
Structure IV

So, there are 5 isomeric esters possible having molecular formula \[{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{2}}}\] .
Hence, the option (B) is the correct answer to the question.
Note: Ester is one of the organic functional groups. The molecular formula of all isomers is the same only the arrangement of bonding atoms is different. Ester contains two types of alkyl groups. One alkyl group bonded to carbonyl carbon and one alkyl bonded to the oxygen atom of the carboxylate group. By varying the alkyl groups we can draw all possible isomers of ester.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




