
The number of mono chloro derivatives of 2-methoxy propane possible are :
a.) 2
b.) 1
c.) 3
d.) 4
Answer
573.3k+ views
Hint: We know that the propane is a three-carbon long chain. The 2- methoxy is the substitution of a methoxy group at the second position of the carbon chain. Then, substituting the Chlorine at all the carbons present in the molecule, we can get the mono chloro derivatives of 2-methoxy propane.
Complete Solution :
- We know that the propane is a three-carbon long chain. The 2- methoxy means at the second carbon, a methoxy group is attached. So, the structure of 2imethoxy propane is as -
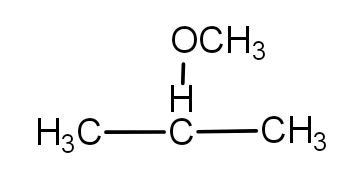
- And further, the mono chloro means, one chlorine group is attached to any carbon of the molecule.
So, our propane chain will be substituted by a chloro group at any unit and a methoxy group at 2nd carbon.
- The isomers are defined as the molecules that have the same molecular formula but the structure is different. Here, this difference will be brought by the chlorine atom.
So, the various possible mono chloro derivatives of 2-methoxy propane are as -
1.) When the Cl group is substituted at first carbon, then the molecule is - 1- chloro -2- methoxy propane. The structure is as -
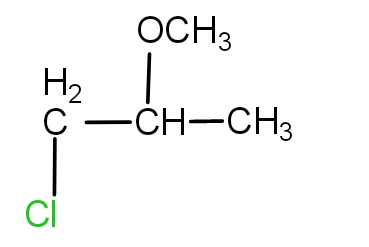
2.) When the Cl group is at second carbon, then the molecule is - 2- chloro -2- methoxy propane. The structure is as -
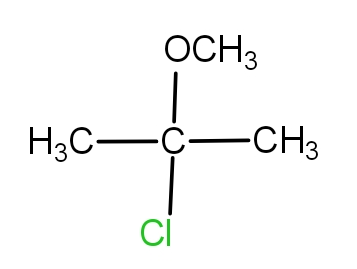
3.) When the Cl group is at methoxy carbon, then the molecule is - 2- chloromethoxy propane. The structure is as -
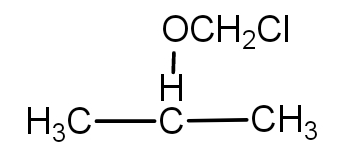
So, the total number of mono chloro derivatives of 2-methoxy propane possible are - 3.
So, the correct answer is “Option C”.
Note: It must be noted that if we place the Cl group on the other carbon that is left i.e. which is on the right hand side, then the molecule will be the same as the 1- chloro -2- methoxy propane. It will not be a new one because, at that time, the numbering would have been started from the right hand side. The two terminal carbons of the 2- methoxy propane are identical.
Complete Solution :
- We know that the propane is a three-carbon long chain. The 2- methoxy means at the second carbon, a methoxy group is attached. So, the structure of 2imethoxy propane is as -

- And further, the mono chloro means, one chlorine group is attached to any carbon of the molecule.
So, our propane chain will be substituted by a chloro group at any unit and a methoxy group at 2nd carbon.
- The isomers are defined as the molecules that have the same molecular formula but the structure is different. Here, this difference will be brought by the chlorine atom.
So, the various possible mono chloro derivatives of 2-methoxy propane are as -
1.) When the Cl group is substituted at first carbon, then the molecule is - 1- chloro -2- methoxy propane. The structure is as -

2.) When the Cl group is at second carbon, then the molecule is - 2- chloro -2- methoxy propane. The structure is as -

3.) When the Cl group is at methoxy carbon, then the molecule is - 2- chloromethoxy propane. The structure is as -

So, the total number of mono chloro derivatives of 2-methoxy propane possible are - 3.
So, the correct answer is “Option C”.
Note: It must be noted that if we place the Cl group on the other carbon that is left i.e. which is on the right hand side, then the molecule will be the same as the 1- chloro -2- methoxy propane. It will not be a new one because, at that time, the numbering would have been started from the right hand side. The two terminal carbons of the 2- methoxy propane are identical.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




