
The number of bonds between sulfur and oxygen atoms in ${{{S}}_2}{{{O}}_8}^{2 - }$ and the number of bonds between sulfur and sulfur atoms in rhombic sulfur, respectively are:
A. $8$ and $6$
B. $8$ and $8$
C. $4$ and $8$
D. $4$ and $4$
Answer
537k+ views
Hint: Sulfur is a chemical element having the symbol ${{S}}$. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ${{{S}}_8}$. Elemental sulfur is a bright yellow crystalline solid at room temperature.
Complete step-by-step answer:
Allotropes are variants of a substance consisting of only one type of an atom. It has a new molecular configuration with new physical properties. Sulfur has various allotropic forms. It can exist in four possible phases:
Two solid polymorphic phases-rhombic sulfur and monoclinic sulfur
Sulfur liquid
Sulfur vapors
Below ${95.6^ \circ }{{C}}$, the stable crystal form is rhombic sulfur. Rhombic sulfur is yellow in color. It is insoluble in water and carbon disulfide. Its density is $2.07{{g}}.{{c}}{{{m}}^{ - 3}}$. The point group of cyclo ${{{S}}_8}$ is ${{{D}}_{{{4d}}}}$. The structure of ${{{S}}_8}$ ring is virtually changed which affects the molecular interactions.
It is large crystal composed of ${{{S}}_8}$ molecules. The eight sulfur atoms in ${{{S}}_8}$ molecule forms a puckered ring. The structure of rhombic sulfur molecule is given below:
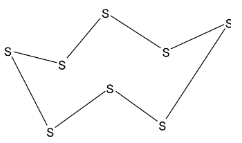
In this molecule, it is obvious that there are eight bonds between two sulfur atoms.
Consider the molecule ${{{H}}_2}{{{S}}_2}{{{O}}_8}$ in which ${{{S}}_2}{{{O}}_8}^{2 - }$ is formed by losing both hydrogen on either sides.

From the above structure, it is obvious that there are six bonds between sulfur and oxygen atoms.
Hence the correct option is B.
Note: Allotropes of sulfur include other ring molecules of sulfur like ${{{S}}_7}{{,}}{{{S}}_{12}}$. Rhombic sulfur melts at a very low temperature because of its simple structure. When it is heated more strongly, the rings break and join together to make long chains. It becomes sticky. When it evaporates, it forms a diatomic sulfur molecule.
Complete step-by-step answer:
Allotropes are variants of a substance consisting of only one type of an atom. It has a new molecular configuration with new physical properties. Sulfur has various allotropic forms. It can exist in four possible phases:
Two solid polymorphic phases-rhombic sulfur and monoclinic sulfur
Sulfur liquid
Sulfur vapors
Below ${95.6^ \circ }{{C}}$, the stable crystal form is rhombic sulfur. Rhombic sulfur is yellow in color. It is insoluble in water and carbon disulfide. Its density is $2.07{{g}}.{{c}}{{{m}}^{ - 3}}$. The point group of cyclo ${{{S}}_8}$ is ${{{D}}_{{{4d}}}}$. The structure of ${{{S}}_8}$ ring is virtually changed which affects the molecular interactions.
It is large crystal composed of ${{{S}}_8}$ molecules. The eight sulfur atoms in ${{{S}}_8}$ molecule forms a puckered ring. The structure of rhombic sulfur molecule is given below:

In this molecule, it is obvious that there are eight bonds between two sulfur atoms.
Consider the molecule ${{{H}}_2}{{{S}}_2}{{{O}}_8}$ in which ${{{S}}_2}{{{O}}_8}^{2 - }$ is formed by losing both hydrogen on either sides.

From the above structure, it is obvious that there are six bonds between sulfur and oxygen atoms.
Hence the correct option is B.
Note: Allotropes of sulfur include other ring molecules of sulfur like ${{{S}}_7}{{,}}{{{S}}_{12}}$. Rhombic sulfur melts at a very low temperature because of its simple structure. When it is heated more strongly, the rings break and join together to make long chains. It becomes sticky. When it evaporates, it forms a diatomic sulfur molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




