
The $ {\text{N - N}} $ bond length is expected to be highest in which of the following compounds gaseous form:
(A) $ {{\text{N}}_{\text{2}}}{\text{O}} $
(B) $ {{\text{N}}_{\text{2}}}{{\text{O}}_5} $
(C) $ {{\text{N}}_{\text{2}}}{{\text{O}}_3} $
(D)
 $ {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} $
$ {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} $

Answer
537.6k+ views
Hint :The $ {\text{N - N}} $ bond length is the average distance between the nuclei of the two bonded nitrogen atoms in the molecule. The bond lengths can be determined from the structures of the molecules. The $ {\text{N - N}} $ bond length will be highest for that molecule for which the average distance between the two bonded nitrogen atoms is largest.
Complete Step By Step Answer:
Nitrous oxide which has the formula $ {{\text{N}}_{\text{2}}}{\text{O}} $ is known to be a resonance hybrid of two linear and highly polar structures with opposing dipole moments. This structure of nitrous oxide accounts for a very low dipole moment of nitrous oxide which is almost equal to $ 0.1{\text{D}} $ . Hence, nitrous oxide has linear structure as shown below. The $ {\text{N - N}} $ bond length is $ 1.12 $ Angstrom.
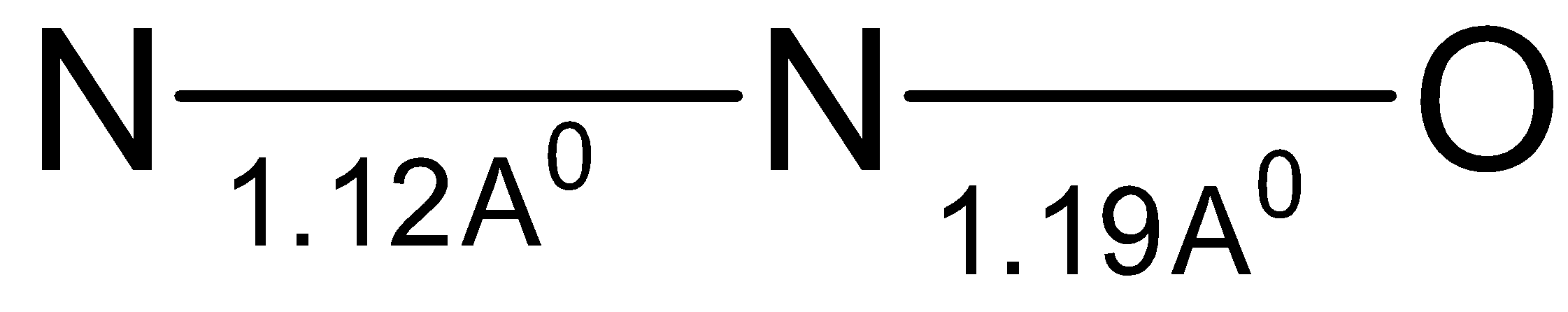
All the group 15 elements have the ability to form hydroxides and peroxides. Nitrogen, being a group 15 element also has the ability to form nitrogen trioxide and nitrogen pentoxide. Nitrogen trioxide has the formula $ {{\text{N}}_{\text{2}}}{{\text{O}}_3} $ . Its structure is not definitely known. Its structure as revealed from the spectroscopic studies is shown below. The $ {\text{N - N}} $ bond length is $ 1.86 $ Angstrom.
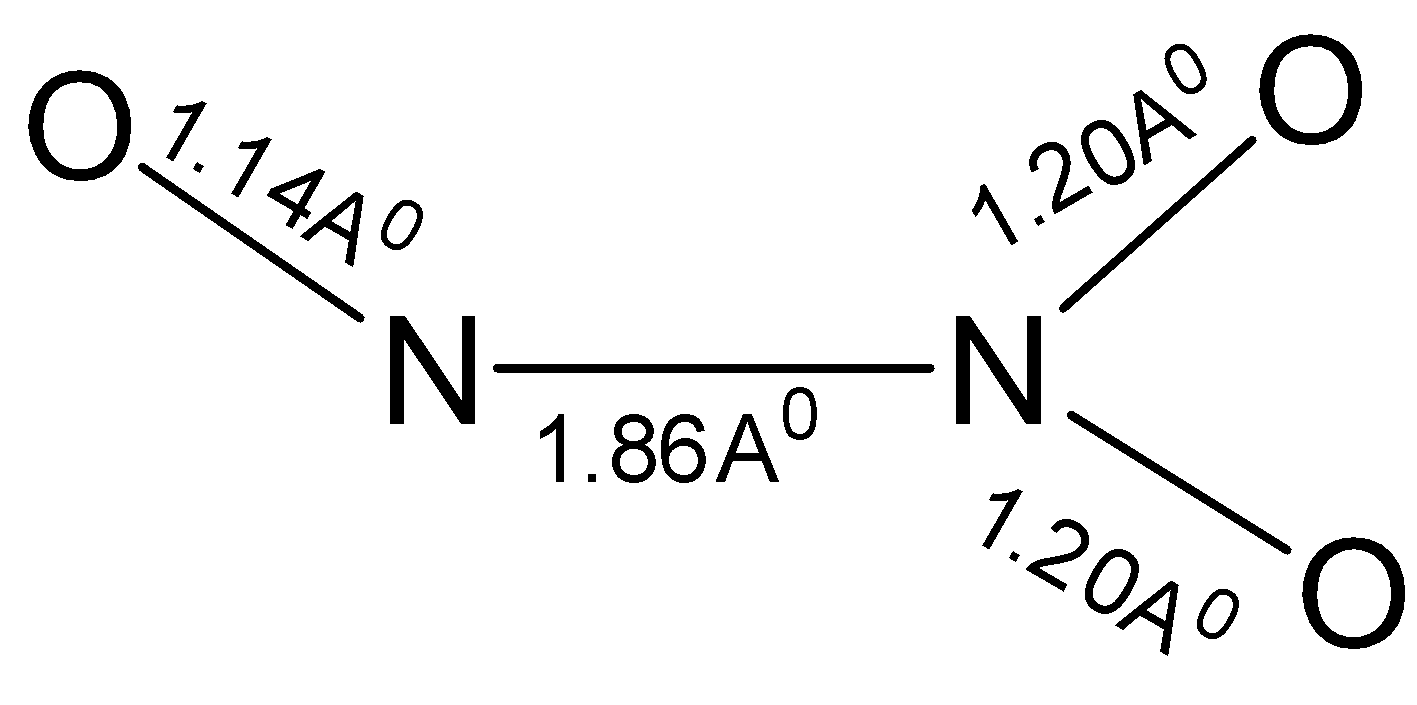
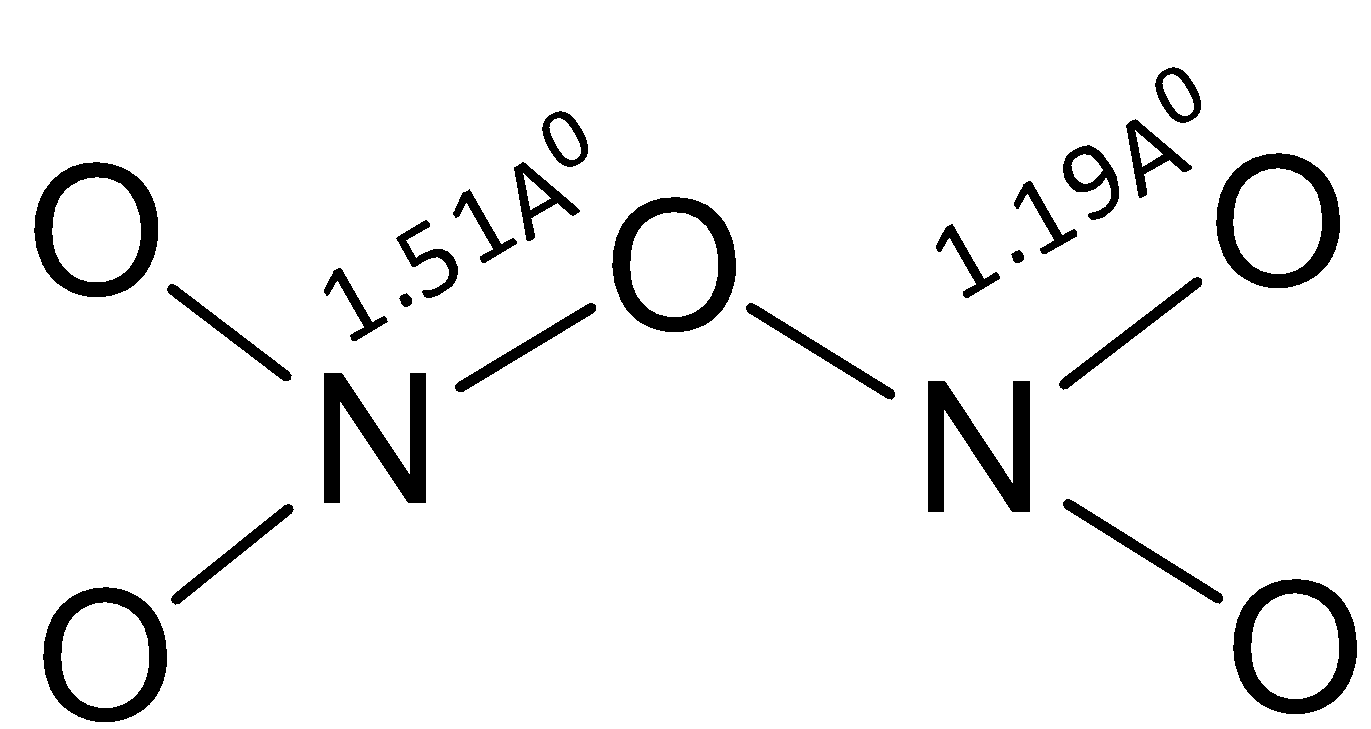
Nitrogen pentoxide has the formula $ {{\text{N}}_{\text{2}}}{{\text{O}}_5} $ has a simple structure and this can be represented as shown below. It does not have any $ {\text{N - N}} $ bond and all the terminal $ {\text{NO}} $ bond lengths are equal due to the resonance between $ {\text{N = O}} $ and $ {\text{N}} \to {\text{O}} $ bonds in the molecule.
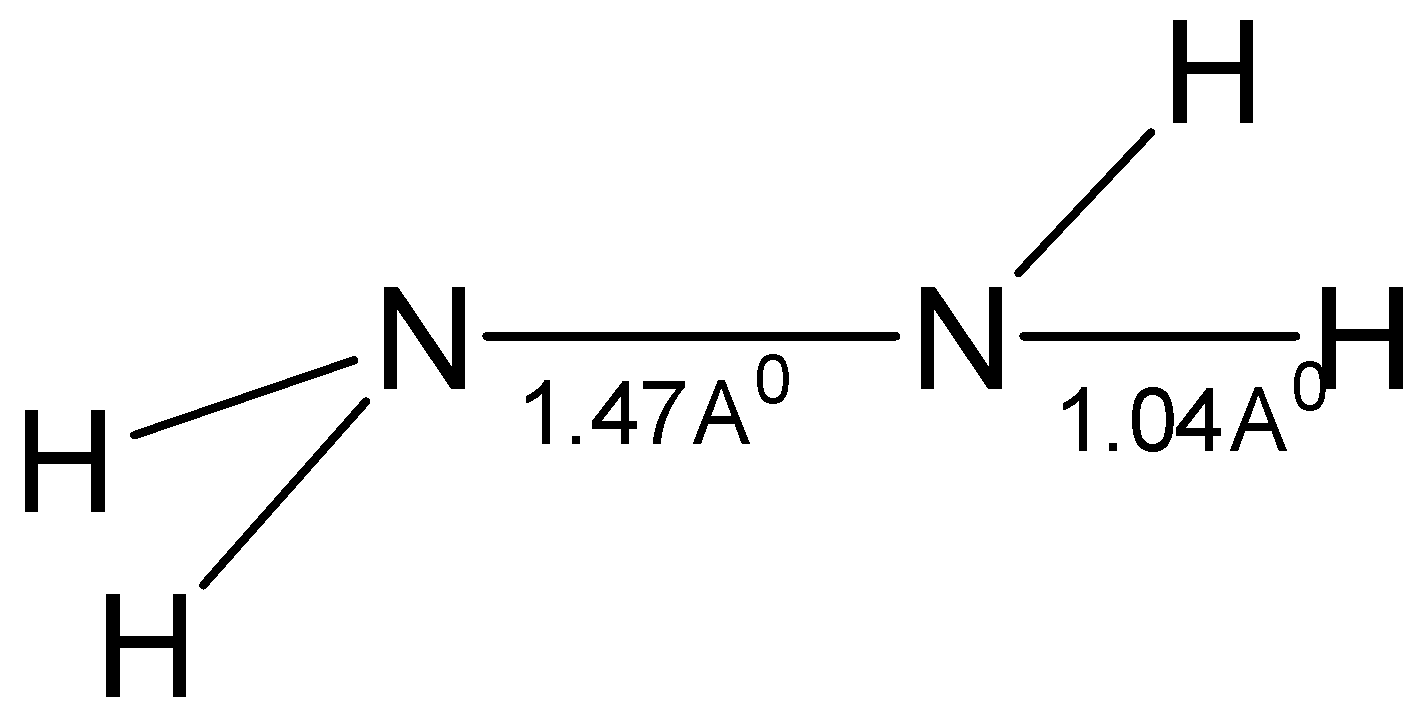
Hydrazine has the formula $ {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} $ and it structurally similar to hydrogen peroxide in the fact that both hydrogen peroxide and hydrazine exist in gauche forms at room temperature. The plane which contains $ {\text{N - N}} $ bond and one of the $ {\text{N - H}} $ bonds of one nitrogen atom is at an angle of $ 95^\circ $ to the plane which has the $ {\text{N - N}} $ bond and one of the $ {\text{N - H}} $ bonds of the other nitrogen atom. The $ {\text{N - N}} $ bond length is $ 1.47 $ Angstrom.
Hence, the $ {\text{N - N}} $ bond length is highest in $ {{\text{N}}_{\text{2}}}{{\text{O}}_3} $ and so the correct answer is C.
Note :
When nitrous oxide is inhaled in moderate quantities, it produces hysterical laughter and hence it is also known as laughing gas. It acts as an anaesthetic in small amounts. It is commercially used as propellant gas in cream bombs.
Hydrazine and some of its alkyl derivatives are used as rocket fuels because hydrazine burns rapidly and completely in air with the evolution of a large amount of heat. Hydrazine is also used as a reagent in organic chemistry.
Complete Step By Step Answer:
Nitrous oxide which has the formula $ {{\text{N}}_{\text{2}}}{\text{O}} $ is known to be a resonance hybrid of two linear and highly polar structures with opposing dipole moments. This structure of nitrous oxide accounts for a very low dipole moment of nitrous oxide which is almost equal to $ 0.1{\text{D}} $ . Hence, nitrous oxide has linear structure as shown below. The $ {\text{N - N}} $ bond length is $ 1.12 $ Angstrom.
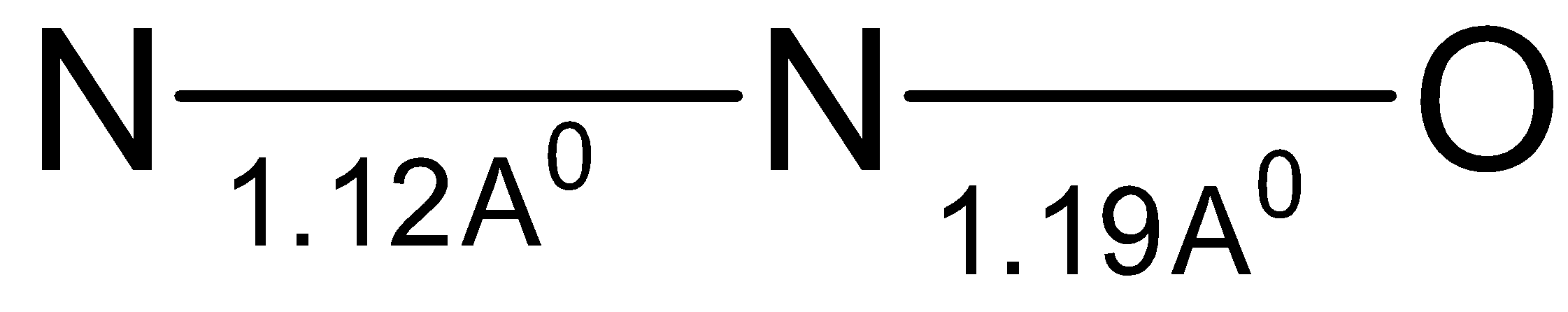
All the group 15 elements have the ability to form hydroxides and peroxides. Nitrogen, being a group 15 element also has the ability to form nitrogen trioxide and nitrogen pentoxide. Nitrogen trioxide has the formula $ {{\text{N}}_{\text{2}}}{{\text{O}}_3} $ . Its structure is not definitely known. Its structure as revealed from the spectroscopic studies is shown below. The $ {\text{N - N}} $ bond length is $ 1.86 $ Angstrom.
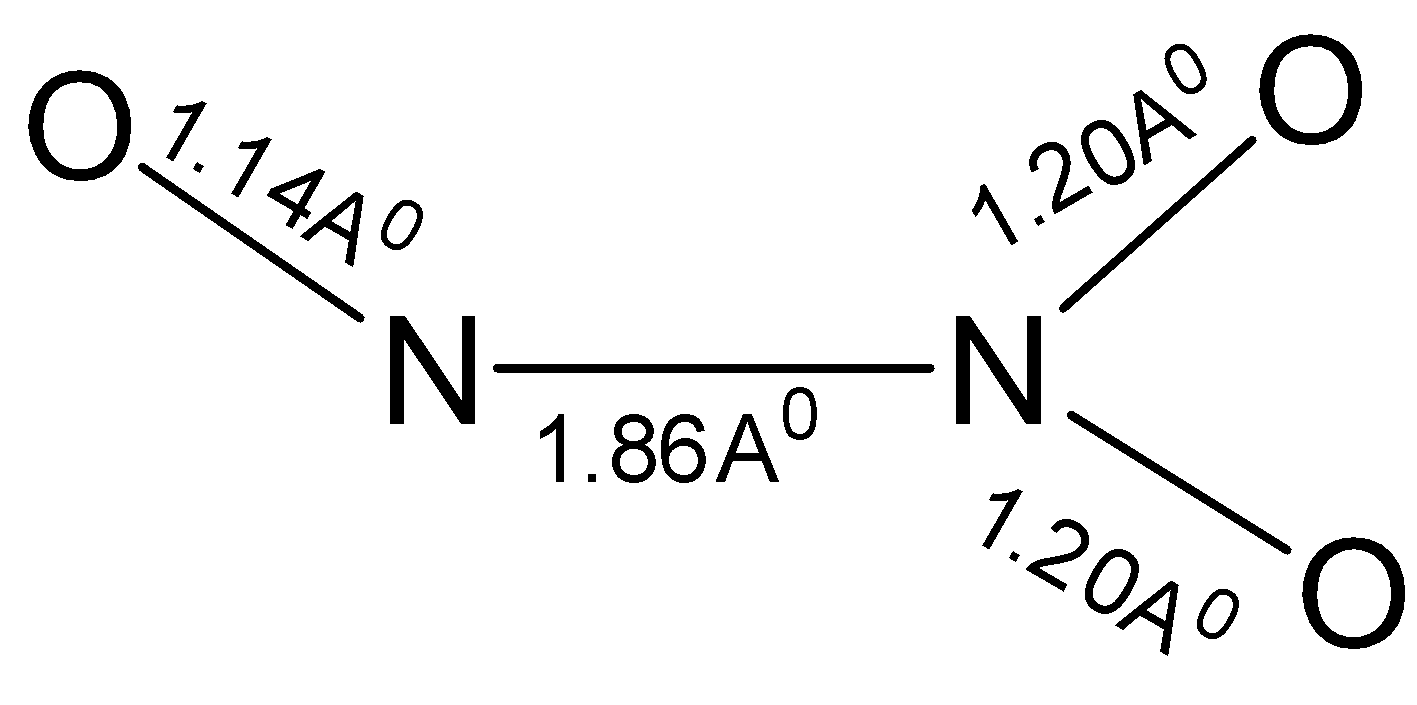
Nitrogen pentoxide has the formula $ {{\text{N}}_{\text{2}}}{{\text{O}}_5} $ has a simple structure and this can be represented as shown below. It does not have any $ {\text{N - N}} $ bond and all the terminal $ {\text{NO}} $ bond lengths are equal due to the resonance between $ {\text{N = O}} $ and $ {\text{N}} \to {\text{O}} $ bonds in the molecule.
Hydrazine has the formula $ {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} $ and it structurally similar to hydrogen peroxide in the fact that both hydrogen peroxide and hydrazine exist in gauche forms at room temperature. The plane which contains $ {\text{N - N}} $ bond and one of the $ {\text{N - H}} $ bonds of one nitrogen atom is at an angle of $ 95^\circ $ to the plane which has the $ {\text{N - N}} $ bond and one of the $ {\text{N - H}} $ bonds of the other nitrogen atom. The $ {\text{N - N}} $ bond length is $ 1.47 $ Angstrom.

Hence, the $ {\text{N - N}} $ bond length is highest in $ {{\text{N}}_{\text{2}}}{{\text{O}}_3} $ and so the correct answer is C.
Note :
When nitrous oxide is inhaled in moderate quantities, it produces hysterical laughter and hence it is also known as laughing gas. It acts as an anaesthetic in small amounts. It is commercially used as propellant gas in cream bombs.
Hydrazine and some of its alkyl derivatives are used as rocket fuels because hydrazine burns rapidly and completely in air with the evolution of a large amount of heat. Hydrazine is also used as a reagent in organic chemistry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




