
The most reactive towards $S{N_1}$ is :
( A ) $PhC{H_2}Cl$
( B ) $PhCl$
( C )$PhCHCl(C{H_3})$
( D )$P - N{O_2}{C_6}{H_4}C{H_2}Cl$
Answer
546.6k+ views
Hint: Order of reactivity towards $S{N_1}$ reaction depends on the formation of intermediate carbocation and another factor is degree of carbocation formed.
Complete step-by-step answer:$S{N_1}$reaction is the nucleophilic substitution reaction . They are the unimolecular reaction because the rate of $S{N_1}$ reaction depends only on the concentration of one reactant.
Order of reactivity of $S{N_1}$ nucleophilic substitution reaction depends on the degree of carbocation i.e; $3^\circ > 2^\circ > 1^\circ $.
Let's check the above given options :
( A ) $PhC{H_2}Cl$ $ \to $
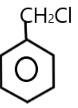
The carbocation of this structure is $1^\circ $ . As we know that $1^\circ $ carbocation is less reactive towards $S{N_1}$ nucleophilic reaction .
( B ) $PhCl$$ \to $
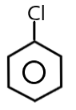
The carbocation of this structure is $2^\circ $. So as we know that $2^\circ $ carbocation is more reactive towards $S{N_1}$ nucleophilic reaction than the $1^\circ $ carbocation .
( C ) $PhCHCl(C{H_3})$ $ \to $
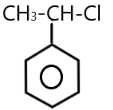
The carbocation of this structure is $2^\circ $ . So $2^\circ $ carbocation is more reactive towards $S{N_1}$ nucleophilic reaction than the $1^\circ $ carbocation . The presence of methyl $ - C{H_3}$ group also increases the positive charge present on the central carbon atom .
( D ) $p - N{O_2}{C_6}{H_4}C{H_2}Cl$ $ \to $
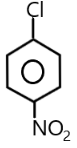
The carbocation of this structure is $1^\circ $. As we know that $1^\circ $ carbocation is least reactive towards $S{N_1}$ nucleophilic substitution reaction . The presence of an electron withdrawing group at the para position of the phenyl group also decreases the positive charge on carbocation .
So, from the above explanations we can conclude that the CORRECT answer of the above question is OPTION ( C ) .
Note: We have to remember that the order of reactivity of $S{N_1}$ nucleophilic substitution reaction depends on the degree of carbocation . Also presence of electron withdrawing group and electron donating group contributes in the reactivity towards $S{N_1}$ nucleophilic substitution reaction .
Complete step-by-step answer:$S{N_1}$reaction is the nucleophilic substitution reaction . They are the unimolecular reaction because the rate of $S{N_1}$ reaction depends only on the concentration of one reactant.
Order of reactivity of $S{N_1}$ nucleophilic substitution reaction depends on the degree of carbocation i.e; $3^\circ > 2^\circ > 1^\circ $.
Let's check the above given options :
( A ) $PhC{H_2}Cl$ $ \to $

The carbocation of this structure is $1^\circ $ . As we know that $1^\circ $ carbocation is less reactive towards $S{N_1}$ nucleophilic reaction .
( B ) $PhCl$$ \to $

The carbocation of this structure is $2^\circ $. So as we know that $2^\circ $ carbocation is more reactive towards $S{N_1}$ nucleophilic reaction than the $1^\circ $ carbocation .
( C ) $PhCHCl(C{H_3})$ $ \to $

The carbocation of this structure is $2^\circ $ . So $2^\circ $ carbocation is more reactive towards $S{N_1}$ nucleophilic reaction than the $1^\circ $ carbocation . The presence of methyl $ - C{H_3}$ group also increases the positive charge present on the central carbon atom .
( D ) $p - N{O_2}{C_6}{H_4}C{H_2}Cl$ $ \to $

The carbocation of this structure is $1^\circ $. As we know that $1^\circ $ carbocation is least reactive towards $S{N_1}$ nucleophilic substitution reaction . The presence of an electron withdrawing group at the para position of the phenyl group also decreases the positive charge on carbocation .
So, from the above explanations we can conclude that the CORRECT answer of the above question is OPTION ( C ) .
Note: We have to remember that the order of reactivity of $S{N_1}$ nucleophilic substitution reaction depends on the degree of carbocation . Also presence of electron withdrawing group and electron donating group contributes in the reactivity towards $S{N_1}$ nucleophilic substitution reaction .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




