
The monomer of nylon-6 is:
A. Cyclohexane
B. Caprolactam
C. Ethylene glycol
D. Amino acid
Answer
600k+ views
Hint: To answer this solution we should know that structurally, nylon-6 is like nylon-6,6 but nylon-6 is made up of one kind of monomer. We should know that nylon 6, 6 is made from two monomers, adipoyl chloride and hexamethylenediamine.
Step by step solution:
We should first know about nylon 6, which is used in making different products. We produce Nylon 6 in two general product types: the regular type for textile uses and the high-strength type for industrial uses. We use nylon 6 mostly in the production of fibre yarns for the manufacture of carpets, tire cords, apparel, hosiery, upholstery, seat belts, parachutes, ropes, and industrial cords.
Now, we will observe each option and give the correct answer about the monomer of nylon 6.
First we will see about cyclohexane. We should know that cyclohexane is cycloalkane with the molecular formula $C_6H_{12}$. We use cyclohexane industrially in the production of adipic acid and caprolactam. It is interesting to note that adipic acid and caprolactam is used in the production of nylon-6,6 and nylon-6 respectively. So, from this discussion we should note that cyclohexane is not a monomer of nylon-6. But it is a pre product of nylon-6.
Now, we will see a second option that is caprolactam. From the above paragraph we know that caprolactum is a monomer of nylon-6. Let us confirm this by observing a reaction.
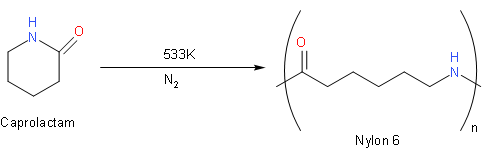
The above reaction is the transformation of monomer caprolactam into polymer Nylon-6. We should know that almost all caprolactam produced goes into the manufacture of Nylon 6. This type of polymerisation is ring-opening polymerization (ROP). We should know that ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer.
Now, we will check another option that is ethylene glycol. We should know that polyethylene glycol (PEG) is formed from several repeating units of its monomer, ethylene glycol (\[{{C}_{2}}{{H}_{6}}{{O}_{2}}\]). We should know that it is a compound with many applications, from industrial manufacturing to medicine.
The fourth option is amino acid; we should know that amino acids are the monomers that make up proteins. We should also note that they join together to form short polymer chains called peptides or longer chains called either polypeptides or proteins.
So, from the above discussion now we know that the correct monomer of nylon-6 is caprolactam that is option B.
Note: We should be careful in handling caprolactam because caprolactam is an irritant and is mildly toxic. it was included on the list of hazardous air pollutants. We should note that if we add caprolactam to water, it hydrolyzes to aminocaproic acid, which is used medicinally.
Step by step solution:
We should first know about nylon 6, which is used in making different products. We produce Nylon 6 in two general product types: the regular type for textile uses and the high-strength type for industrial uses. We use nylon 6 mostly in the production of fibre yarns for the manufacture of carpets, tire cords, apparel, hosiery, upholstery, seat belts, parachutes, ropes, and industrial cords.
Now, we will observe each option and give the correct answer about the monomer of nylon 6.
First we will see about cyclohexane. We should know that cyclohexane is cycloalkane with the molecular formula $C_6H_{12}$. We use cyclohexane industrially in the production of adipic acid and caprolactam. It is interesting to note that adipic acid and caprolactam is used in the production of nylon-6,6 and nylon-6 respectively. So, from this discussion we should note that cyclohexane is not a monomer of nylon-6. But it is a pre product of nylon-6.
Now, we will see a second option that is caprolactam. From the above paragraph we know that caprolactum is a monomer of nylon-6. Let us confirm this by observing a reaction.

The above reaction is the transformation of monomer caprolactam into polymer Nylon-6. We should know that almost all caprolactam produced goes into the manufacture of Nylon 6. This type of polymerisation is ring-opening polymerization (ROP). We should know that ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer.
Now, we will check another option that is ethylene glycol. We should know that polyethylene glycol (PEG) is formed from several repeating units of its monomer, ethylene glycol (\[{{C}_{2}}{{H}_{6}}{{O}_{2}}\]). We should know that it is a compound with many applications, from industrial manufacturing to medicine.
The fourth option is amino acid; we should know that amino acids are the monomers that make up proteins. We should also note that they join together to form short polymer chains called peptides or longer chains called either polypeptides or proteins.
So, from the above discussion now we know that the correct monomer of nylon-6 is caprolactam that is option B.
Note: We should be careful in handling caprolactam because caprolactam is an irritant and is mildly toxic. it was included on the list of hazardous air pollutants. We should note that if we add caprolactam to water, it hydrolyzes to aminocaproic acid, which is used medicinally.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




