
The molecule that has a linear structure is:
(A). $C{O_2}$
(B). $N{O_2}$
(C). $S{O_2}$
(D). $Si{O_2}$
Answer
575.7k+ views
Hint: The three dimensional shapes of the many small molecules are often predicted by applying the valence shell electron pair repulsion theory (VSEPR) when atoms combine to make molecules, pair of valence electrons arrange themselves as far away from one another as possible. Differently to characterize molecular shape is in terms of hybrid orbitals.
Complete answer:
Linear molecules may be molecules during which atoms are deployed in a straight line (under $180^\circ $ ange.) have $sp$ hybridization at the central atom .
For example $ \Rightarrow C{O_2}$
$O = C = O$
Linear structure
-$N{O_2} \Rightarrow $ It has bent shape
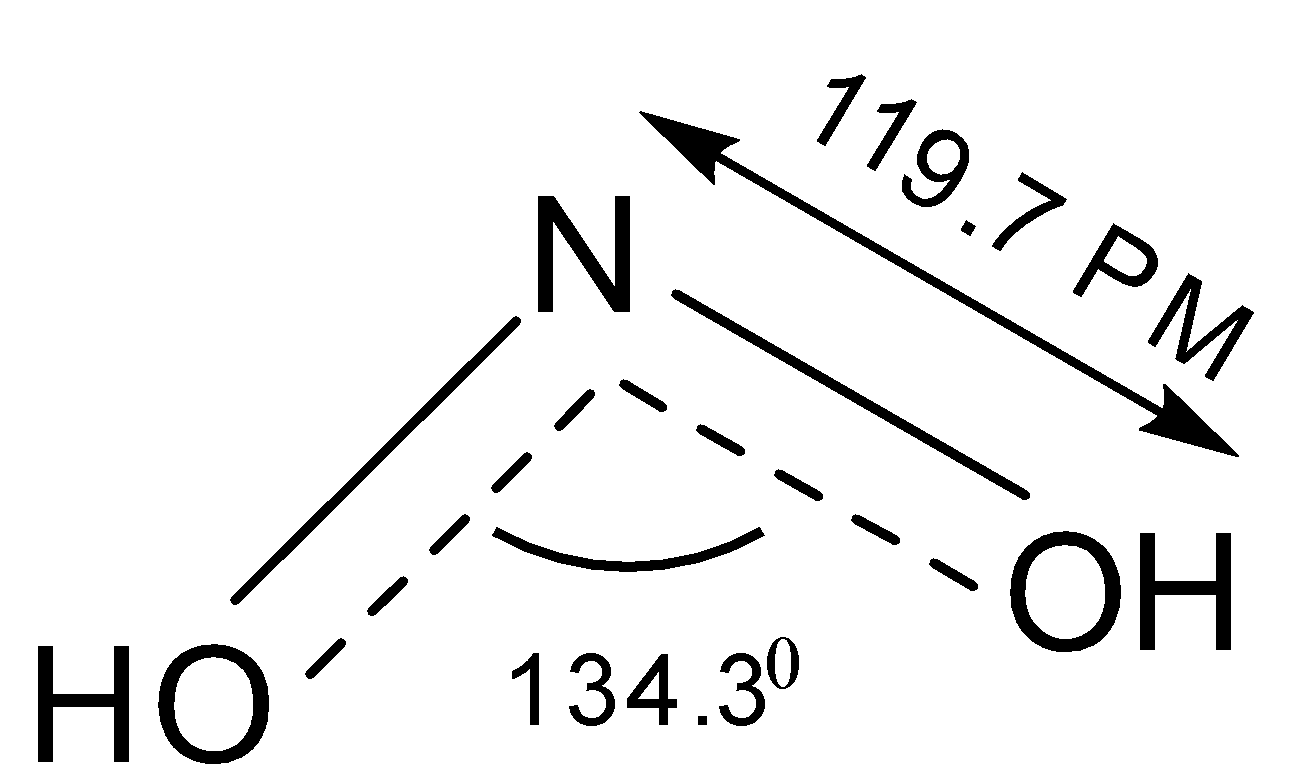
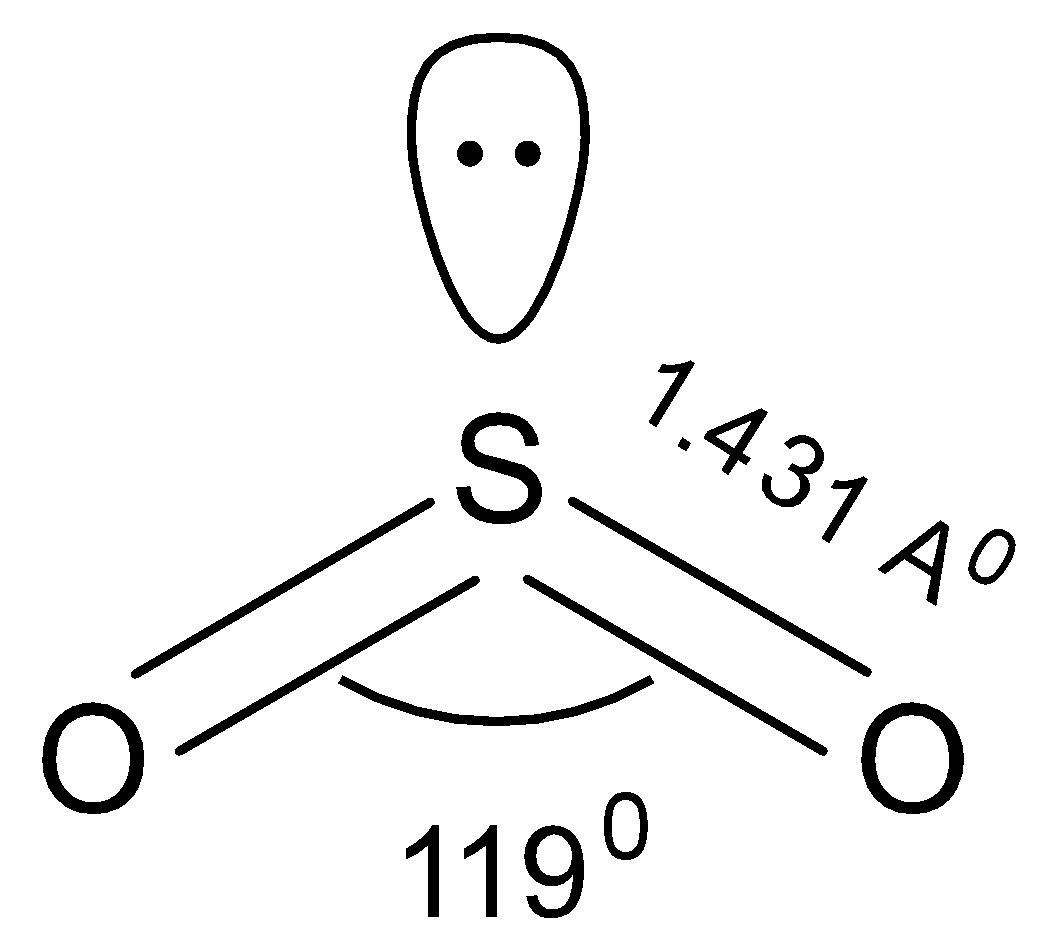
-The molecular shape of $S{O_2}$ is V-shaped or bent shape.
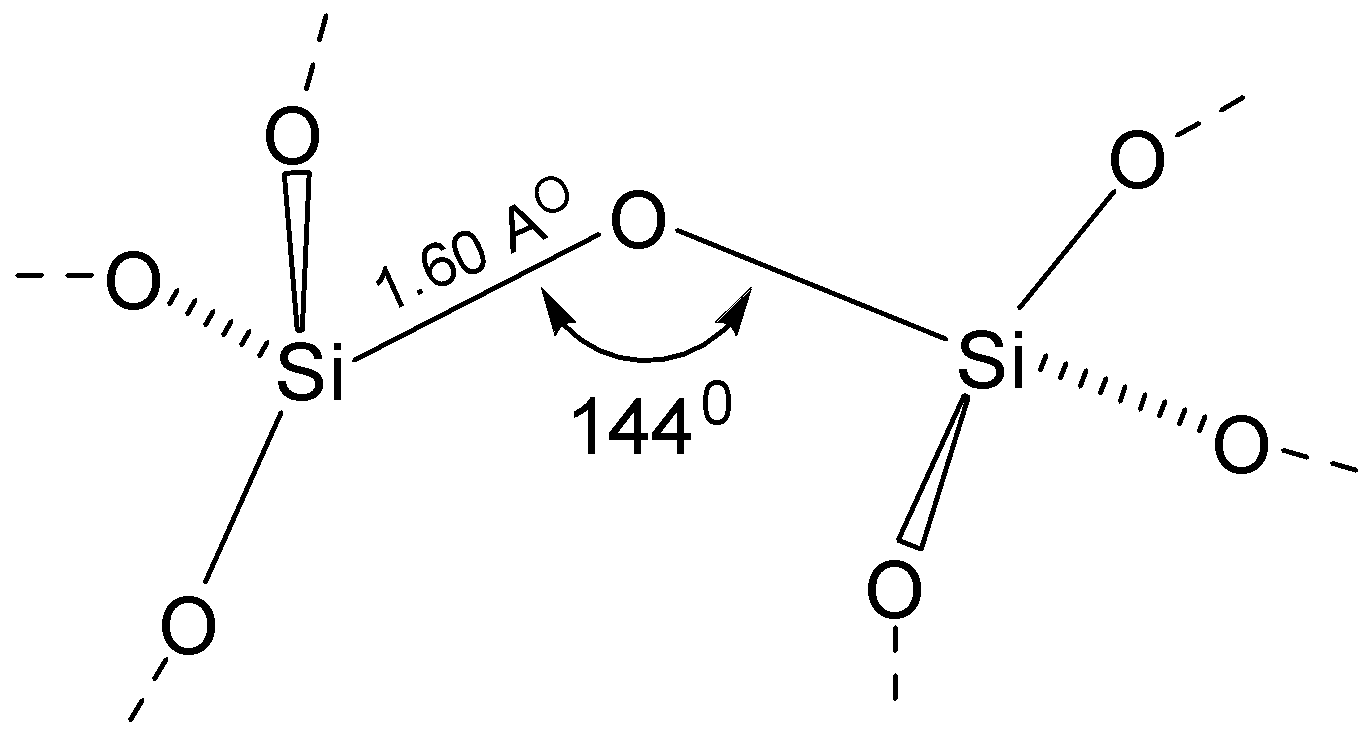
-$Si{O_2}$ , silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central silicon atom
Hence option A is correct .
Additional information :
Though VSEPR theory is able to explain the shapes of simple molecules but it is unable to predict the shape in a number of cases . Thus , it had a limited application only . Moreover , talking of the direction of electron pairs does not seem to be very rational . Thus , Lewis' approach as well as VSEPR theory had a number of limitations .
To explain the above limitations , two important theories called modern theories of covalent bond formation have been put forward , that is the valence bond theory and the molecular orbital theory .
Note:
While predicting the geometry of molecules containing double bonds , it should be kept in mind that the double bond is considered one electron pair . For example , in case of ozone the central oxygen atom is considered to have two bond pairs and one lone pair and has a bent shape structure .
Complete answer:
Linear molecules may be molecules during which atoms are deployed in a straight line (under $180^\circ $ ange.) have $sp$ hybridization at the central atom .
For example $ \Rightarrow C{O_2}$
$O = C = O$
Linear structure
-$N{O_2} \Rightarrow $ It has bent shape

-The molecular shape of $S{O_2}$ is V-shaped or bent shape.

-$Si{O_2}$ , silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central silicon atom

Hence option A is correct .
Additional information :
Though VSEPR theory is able to explain the shapes of simple molecules but it is unable to predict the shape in a number of cases . Thus , it had a limited application only . Moreover , talking of the direction of electron pairs does not seem to be very rational . Thus , Lewis' approach as well as VSEPR theory had a number of limitations .
To explain the above limitations , two important theories called modern theories of covalent bond formation have been put forward , that is the valence bond theory and the molecular orbital theory .
Note:
While predicting the geometry of molecules containing double bonds , it should be kept in mind that the double bond is considered one electron pair . For example , in case of ozone the central oxygen atom is considered to have two bond pairs and one lone pair and has a bent shape structure .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




