
The molecular weight of acetone is M. The molecular weight of diacetone alcohol is:
Answer
567k+ views
Hint:When two molecules of acetone combine then one mole of diacetone alcohol is formed. The molecular mass on both sides will remain the same.
Complete step by step answer:
When two molecules of acetone react they form diacetone alcohol. The reaction occurs as:
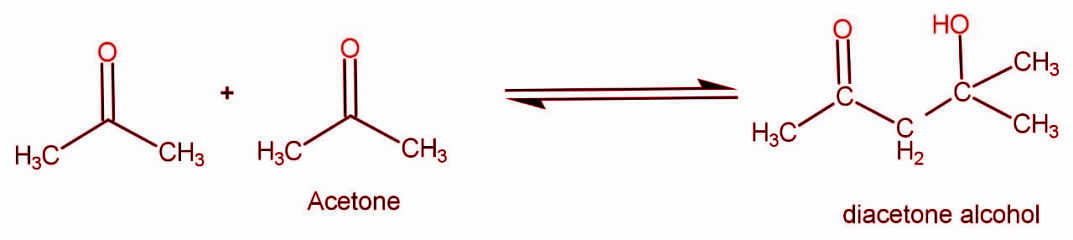
The molecular formula for acetone is \[{{\text{C}}_3}{{\text{H}}_6}{\text{O}}\] . The two molecules are reacting so the formula will get doubled. We can write the formula for reactants species as \[{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_2}\] .
The general formula for product that is diacetone alcohol is \[{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_2}\] .
So if the molecular mass of 1 mole of acetone is M and 2 moles of acetone reacts, so the molecular mass of the product formed will be 2M.
Additional information:
Acetone is the basic ketone, it is the first ketone. It is colourless and very highly volatile. It is a flammable liquid. It is a pungent smelling liquid. It is highly soluble in water and also acts as solvent for various organic compounds. We usually find acetone in nail paint remover and as a thinner in paint. Acetone is also present in the human body especially produced in high quantities by people suffering from diabetes. One third of the world's acetone production is used as a solvent for the formation of synthetic fibers.
Note:
Diacetone alcohol is an organic compound and is commonly known as DDA. It is used as solvent, preservative, cellulose ester lacquers, to make artificial silk and leather, coating for paper and textile, permanent markers, dopes and thinners. It is used as an intermediate for the production of other compounds. The IUPAC name for DDA is 4-hydroxy-4methylpentan-2-one.
Complete step by step answer:
When two molecules of acetone react they form diacetone alcohol. The reaction occurs as:
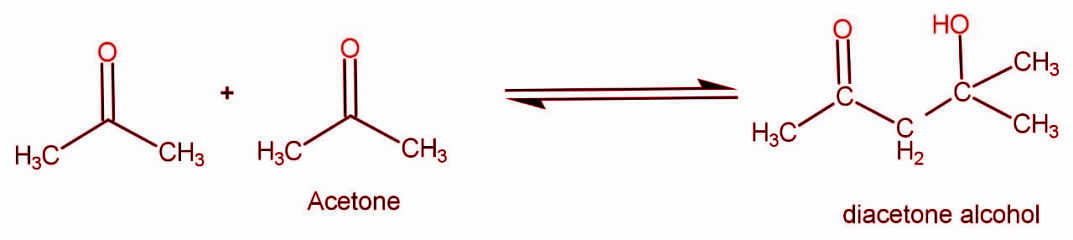
The molecular formula for acetone is \[{{\text{C}}_3}{{\text{H}}_6}{\text{O}}\] . The two molecules are reacting so the formula will get doubled. We can write the formula for reactants species as \[{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_2}\] .
The general formula for product that is diacetone alcohol is \[{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_2}\] .
So if the molecular mass of 1 mole of acetone is M and 2 moles of acetone reacts, so the molecular mass of the product formed will be 2M.
Additional information:
Acetone is the basic ketone, it is the first ketone. It is colourless and very highly volatile. It is a flammable liquid. It is a pungent smelling liquid. It is highly soluble in water and also acts as solvent for various organic compounds. We usually find acetone in nail paint remover and as a thinner in paint. Acetone is also present in the human body especially produced in high quantities by people suffering from diabetes. One third of the world's acetone production is used as a solvent for the formation of synthetic fibers.
Note:
Diacetone alcohol is an organic compound and is commonly known as DDA. It is used as solvent, preservative, cellulose ester lacquers, to make artificial silk and leather, coating for paper and textile, permanent markers, dopes and thinners. It is used as an intermediate for the production of other compounds. The IUPAC name for DDA is 4-hydroxy-4methylpentan-2-one.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




