
The molecular formula ${C_3}{H_9}N$ cannot represent :
( A ) $1^\circ $ amine
( B ) $2^\circ $ amine
( C ) $3^\circ $ amine
( D ) Quaternary salt
Answer
548.1k+ views
Hint: There are only $3 - C$ atoms present in the molecular formula so only those structures are possible according to the formula which are having $3 - C$ atom. Quaternary salt requires $4 - C$ atoms .
Complete step-by-step answer:First let’s see the structural isomers of this molecular formula :
1. $C{H_3} - C{H_2} - C{H_2} - N{H_2}$$ \to $ $Propan - 1 - a\min e$ ( $1^\circ $ amine )
2. $C{H_3} - NH - C{H_2} - C{H_3}$ $ \to $ \[N - methylethanamine\] ($2^\circ $ amine )
3.
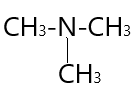 $ \to $ \[N,N-Dimethylmethanamine\;\]( $3^\circ $amine )
$ \to $ \[N,N-Dimethylmethanamine\;\]( $3^\circ $amine )
4.
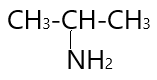 $ \to $\[Propan - 2 - amine\;\] ( $1^\circ $ amine )
$ \to $\[Propan - 2 - amine\;\] ( $1^\circ $ amine )
There are 4 structural isomers of the molecular formula${C_3}{H_9}N$ according to the number of carbon atoms , number of hydrogen atoms and number of nitrogen atoms .
As this molecular formula has $3 - C$atom , $9 - H$ atom , $1 - N$ atom . As we can see that molecular formula has only $3 - C$ atoms so only these 4 structures are possible: $1^\circ $ amine , $2^\circ $ amine , $3^\circ $ amine .
While for Quaternary salt, we require $4 - $carbon atoms ,which according to the molecular formula is not possible.
In ( 1 ) structural isomer $ - N{H_2}$ group attaches to the one carbon atom and results in the $1^\circ $ amine.
In ( 2 ) structural isomers $ - N{H_2}$ group attach to two carbon atoms and result in the formation of $2^\circ $ amine .
In ( 3 ) structural isomer $ - N{H_2}$ group attached to three carbon atoms and result in the formation of $3^\circ $amine .
In ( 4 ) structural isomer $ - N{H_2}$ group attached to one carbon atom and results in the formation of $1^\circ $ amine.
Note:Structural isomers depend on the number of atoms present in the given molecular formula. So we have to count the number of atoms given in the molecular formula .
Complete step-by-step answer:First let’s see the structural isomers of this molecular formula :
1. $C{H_3} - C{H_2} - C{H_2} - N{H_2}$$ \to $ $Propan - 1 - a\min e$ ( $1^\circ $ amine )
2. $C{H_3} - NH - C{H_2} - C{H_3}$ $ \to $ \[N - methylethanamine\] ($2^\circ $ amine )
3.

4.
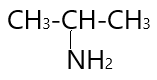
There are 4 structural isomers of the molecular formula${C_3}{H_9}N$ according to the number of carbon atoms , number of hydrogen atoms and number of nitrogen atoms .
As this molecular formula has $3 - C$atom , $9 - H$ atom , $1 - N$ atom . As we can see that molecular formula has only $3 - C$ atoms so only these 4 structures are possible: $1^\circ $ amine , $2^\circ $ amine , $3^\circ $ amine .
While for Quaternary salt, we require $4 - $carbon atoms ,which according to the molecular formula is not possible.
In ( 1 ) structural isomer $ - N{H_2}$ group attaches to the one carbon atom and results in the $1^\circ $ amine.
In ( 2 ) structural isomers $ - N{H_2}$ group attach to two carbon atoms and result in the formation of $2^\circ $ amine .
In ( 3 ) structural isomer $ - N{H_2}$ group attached to three carbon atoms and result in the formation of $3^\circ $amine .
In ( 4 ) structural isomer $ - N{H_2}$ group attached to one carbon atom and results in the formation of $1^\circ $ amine.
Note:Structural isomers depend on the number of atoms present in the given molecular formula. So we have to count the number of atoms given in the molecular formula .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




