
The major product of the following addition reaction is
\[C{{H}_{3}}-CH=C{{H}_{2}}\xrightarrow{C{{l}_{2}}/{{H}_{2}}O}\]
A.
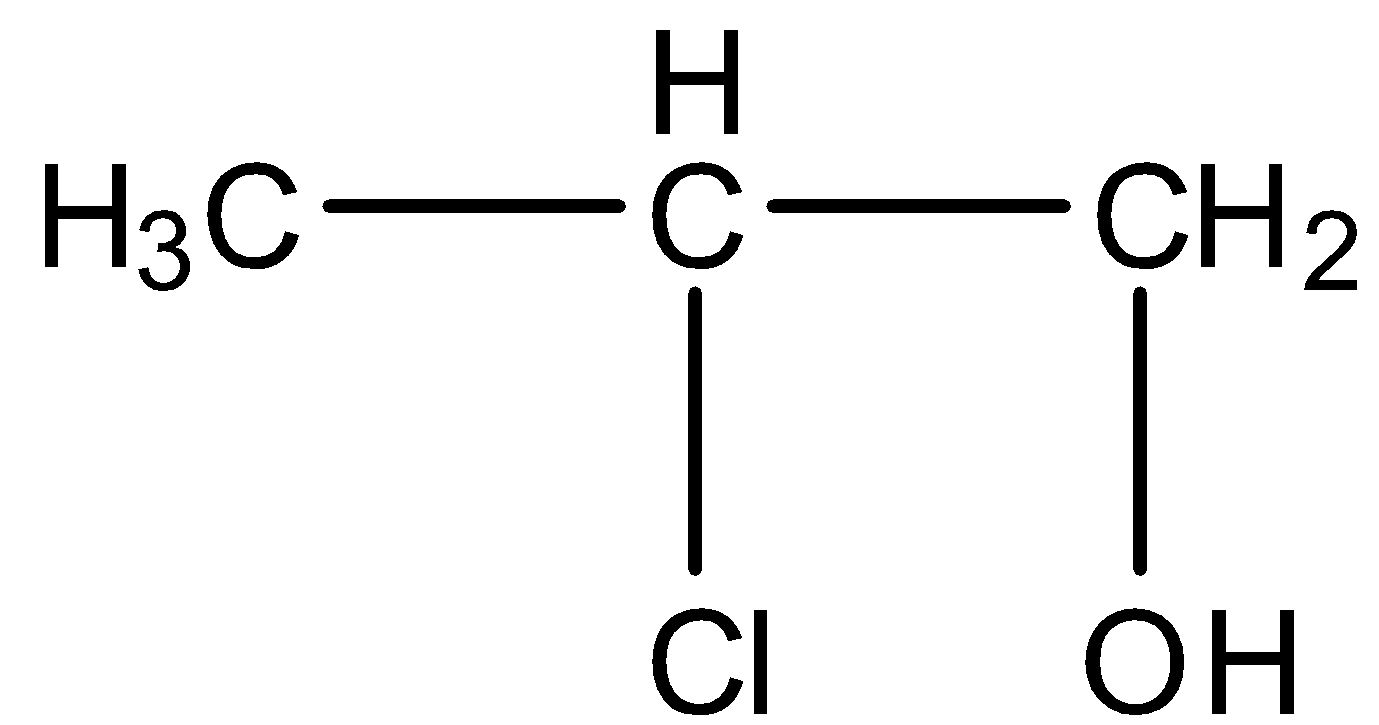
B.
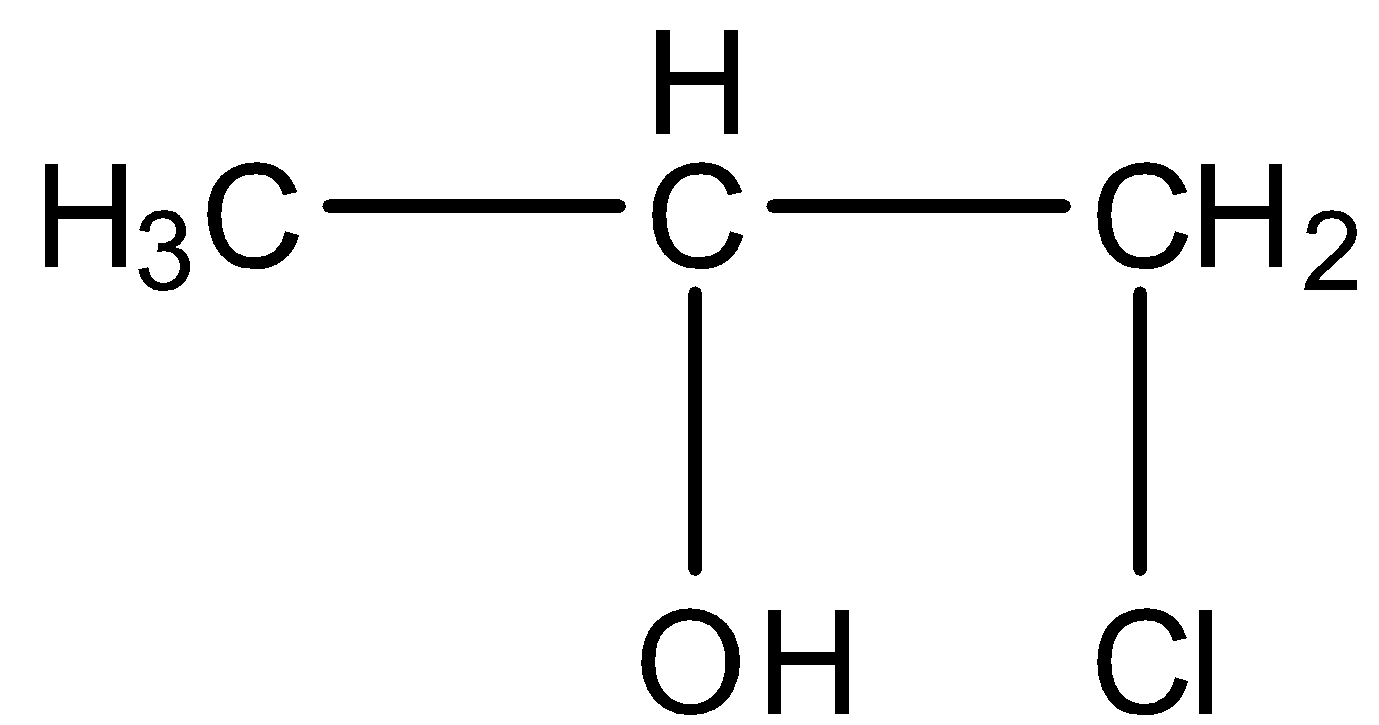
C.
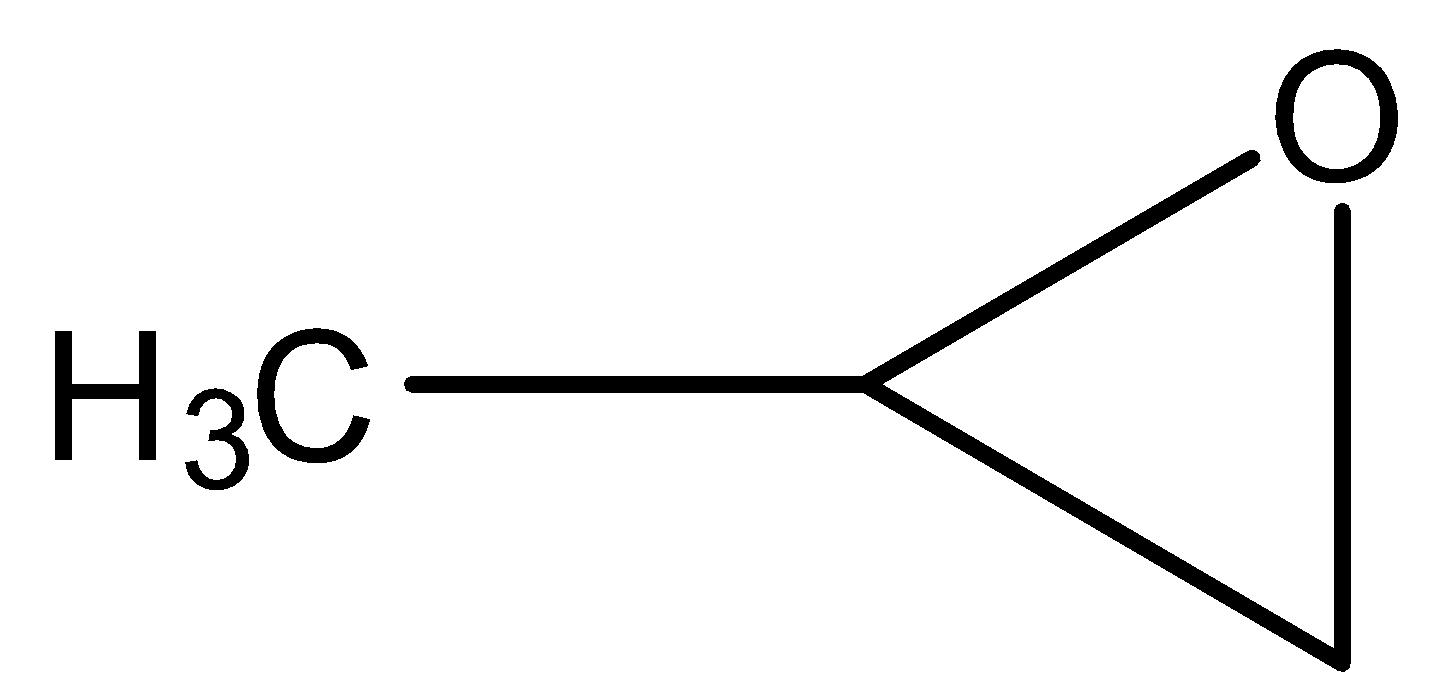
D.




Answer
577.8k+ views
Hint:. Whenever addition reaction is going to happen on alkenes then always the stable form of carbocation formed during the reaction should be either tertiary or secondary carbocation. Formation of primary carbocation during the reaction does not favor the formation of the addition product.
Complete step by step answer:
- In the given question chlorine and water are participating in addition reaction with propene.
- Propene is an unsaturated compound.
- The product formed during the reaction of chlorine and water with propene is as follows.
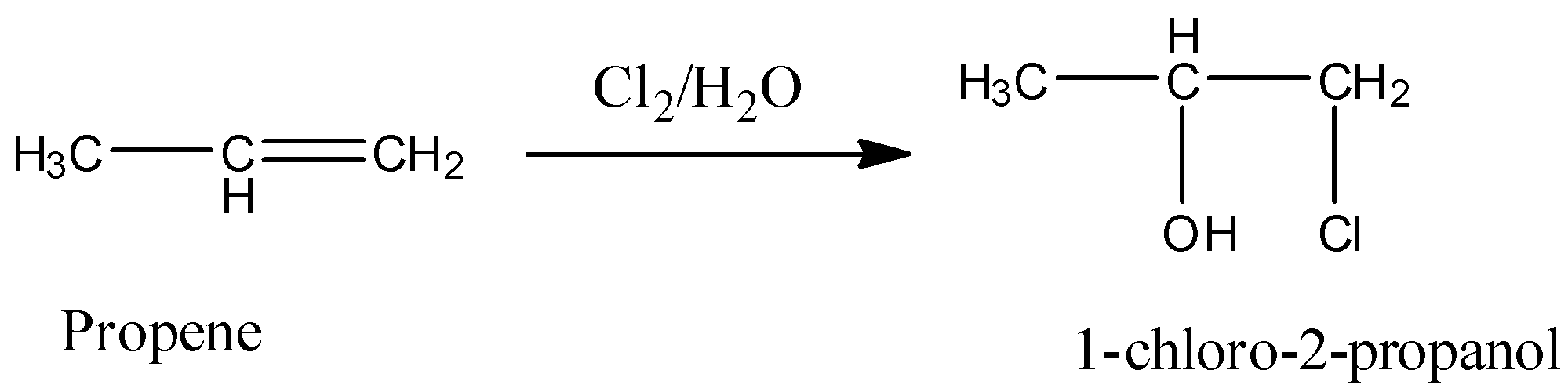
- The proper mechanism of the above reaction is as follows.
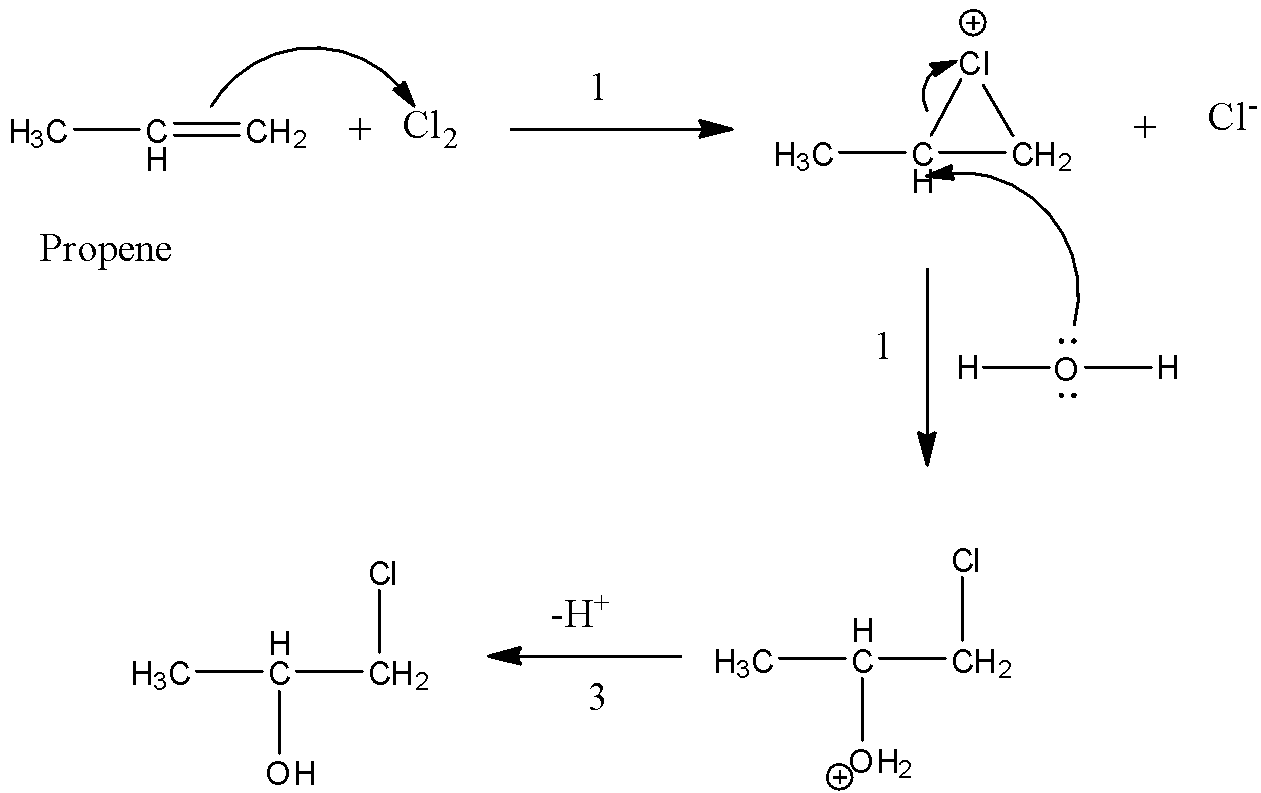
- The above reaction contains three steps.
- In the first step the double bond present in propene reacts with chlorine and forms a three membered ring as the product.
- In the second step the lone pair of electrons present on the oxygen atom of the water molecule reacts with secondary carbon which is present in the compound and cleaves the three membered and forms a stable product after losing a hydrogen atom in third step.
- Therefore the product forms after reaction chlorine and water with propene is 1-chloro-2-propanol.
So, the correct answer is “Option B”.
Note: The unsaturated compound (compound containing double or triple bond in its structure) is converted into a saturated compound at the end of the reaction after reacting with chlorine and water. Then this reaction is called addition reaction.
Conversion of saturated compounds to unsaturated compounds is called an elimination reaction.
Complete step by step answer:
- In the given question chlorine and water are participating in addition reaction with propene.
- Propene is an unsaturated compound.
- The product formed during the reaction of chlorine and water with propene is as follows.

- The proper mechanism of the above reaction is as follows.

- The above reaction contains three steps.
- In the first step the double bond present in propene reacts with chlorine and forms a three membered ring as the product.
- In the second step the lone pair of electrons present on the oxygen atom of the water molecule reacts with secondary carbon which is present in the compound and cleaves the three membered and forms a stable product after losing a hydrogen atom in third step.
- Therefore the product forms after reaction chlorine and water with propene is 1-chloro-2-propanol.
So, the correct answer is “Option B”.
Note: The unsaturated compound (compound containing double or triple bond in its structure) is converted into a saturated compound at the end of the reaction after reacting with chlorine and water. Then this reaction is called addition reaction.
Conversion of saturated compounds to unsaturated compounds is called an elimination reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




