
The magnetic moment of $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ ion on the basis of spin only formula will be ___.
A) $\text{ 1}\text{.232 }$
B) $\text{ 1}\text{.332 }$
C) $\text{ 1}\text{.532 }$
D) $\text{ 1}\text{.732 }$
Answer
583.2k+ views
Hint: The magnetic behaviour of the ion is expressed in terms of the number of unpaired electrons in an element. The magnetic moment is expressed in terms of unpaired electrons as follows:
$\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n (n + 2)}}\text{ B}\text{.M}\text{. }$
Where $\text{ }\!\!\mu\!\!\text{ }$ the magnetic moment and ‘n’ are is the number of the unpaired electron.
Complete step by step answer:
In lanthanides, the magnetic moment arises due to the spin motion of the electron i.e. spin magnetic moment (S) and the orbital motion of the electrons around the nucleus i.e. orbital magnetic moment (L). The magnetic moment is expressed in Bohr magneton abbreviated as BM.
The magnetic moment arises due to the spin of electrons and the orbital motion of the electron. The Bohr magneton is calculated from the following formula:
$\text{ }{{\text{ }\!\!\mu\!\!\text{ }}_{\text{eff}}}\text{ = }\sqrt{\text{4S (S+1)+L(L+1)}}\text{ B}\text{.M}\text{. }$
Where $\text{ }\!\!\mu\!\!\text{ }$ the magnetic moment of the ions is expressed in Bohr's magneton (BM) units.
‘S’ is the resultant spin quantum number and the ‘L’ is the resultant orbital momentum quantum number of electrons.
But, for simplicity, we neglect the orbital momentum of electrons, and the formula is reduced to,
$\text{ }{{\text{ }\!\!\mu\!\!\text{ }}_{\text{eff}}}\text{ = }\sqrt{\text{4S (S+1)}}\text{ B}\text{.M}\text{. }$
The above formula cane is write in terms of the number of unpaired electrons as follows:
$\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n (n + 2)}}\text{ B}\text{.M}\text{. }$
We are interested to find out the magnetic moment of $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ ions. Let’s first write down the electronic configuration of $\text{ Ce }$ the atom.
$\text{ Ce = }\left[ \text{Xe} \right]\text{ 4}{{\text{f}}^{\text{2}}}\text{5}{{\text{d}}^{0}}\text{6}{{\text{s}}^{\text{2}}}\text{ }$
The cerium atoms lose its two electrons from the $\text{ 6s }$ orbital and one electron from the $\text{ 4f }$ orbital. Thus the electronic configuration of the $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ion is as follows,
\[\text{ C}{{\text{e}}^{\text{3+}}}\text{ = }\left[ \text{Xe} \right]\text{ 4}{{\text{f}}^{\text{1}}}\text{5}{{\text{d}}^{0}}\text{4}{{\text{s}}^{0}}\text{ }\]
The valence shell of $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ ion be depicted as follows,
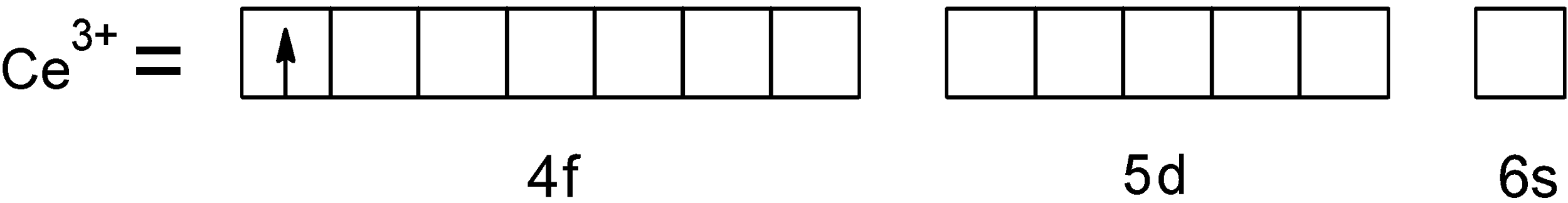
The $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ion has one unpaired electron in the $\text{ 4f }$orbital. Let's apply the magnetic moment formula we have,
$\begin{align}
& \text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n (n + 2)}}\text{ } \\
& \Rightarrow \text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{1 (1 + 2)}}\text{ } \\
& \Rightarrow \text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{ 3}}\text{ } \\
& \therefore \text{ }\!\!\mu\!\!\text{ = 1}\text{.732 B}\text{.M}\text{.} \\
\end{align}$
Therefore, the magnetic moment of $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ ion is equal to $\text{1}\text{.732 B}\text{.M}\text{.}$
So, the correct answer is “Option D”.
Note: Note that, there is a difference in the magnetic moment of the d and f block element. In the case of transition (d) metals the magnetic moment corresponds to the spin only. The magnetic moment arises due to the spin motion of an electron and the orbital motion of an electron. However, in case of d-orbitals are not well shielded from the environment. As a result, the d-electrons of the metal interact strongly with the surrounding ligands. Due to this the orbital motion is quenched and thus the magnetic moment of d-block elements arises only due to the spin motion of the electrons only.
In lanthanides, the orbital motion is not quenched. Thus the effective magnetic moment arises due to spin and orbital motion.
$\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n (n + 2)}}\text{ B}\text{.M}\text{. }$
Where $\text{ }\!\!\mu\!\!\text{ }$ the magnetic moment and ‘n’ are is the number of the unpaired electron.
Complete step by step answer:
In lanthanides, the magnetic moment arises due to the spin motion of the electron i.e. spin magnetic moment (S) and the orbital motion of the electrons around the nucleus i.e. orbital magnetic moment (L). The magnetic moment is expressed in Bohr magneton abbreviated as BM.
The magnetic moment arises due to the spin of electrons and the orbital motion of the electron. The Bohr magneton is calculated from the following formula:
$\text{ }{{\text{ }\!\!\mu\!\!\text{ }}_{\text{eff}}}\text{ = }\sqrt{\text{4S (S+1)+L(L+1)}}\text{ B}\text{.M}\text{. }$
Where $\text{ }\!\!\mu\!\!\text{ }$ the magnetic moment of the ions is expressed in Bohr's magneton (BM) units.
‘S’ is the resultant spin quantum number and the ‘L’ is the resultant orbital momentum quantum number of electrons.
But, for simplicity, we neglect the orbital momentum of electrons, and the formula is reduced to,
$\text{ }{{\text{ }\!\!\mu\!\!\text{ }}_{\text{eff}}}\text{ = }\sqrt{\text{4S (S+1)}}\text{ B}\text{.M}\text{. }$
The above formula cane is write in terms of the number of unpaired electrons as follows:
$\text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n (n + 2)}}\text{ B}\text{.M}\text{. }$
We are interested to find out the magnetic moment of $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ ions. Let’s first write down the electronic configuration of $\text{ Ce }$ the atom.
$\text{ Ce = }\left[ \text{Xe} \right]\text{ 4}{{\text{f}}^{\text{2}}}\text{5}{{\text{d}}^{0}}\text{6}{{\text{s}}^{\text{2}}}\text{ }$
The cerium atoms lose its two electrons from the $\text{ 6s }$ orbital and one electron from the $\text{ 4f }$ orbital. Thus the electronic configuration of the $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ion is as follows,
\[\text{ C}{{\text{e}}^{\text{3+}}}\text{ = }\left[ \text{Xe} \right]\text{ 4}{{\text{f}}^{\text{1}}}\text{5}{{\text{d}}^{0}}\text{4}{{\text{s}}^{0}}\text{ }\]
The valence shell of $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ ion be depicted as follows,

The $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ion has one unpaired electron in the $\text{ 4f }$orbital. Let's apply the magnetic moment formula we have,
$\begin{align}
& \text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{n (n + 2)}}\text{ } \\
& \Rightarrow \text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{1 (1 + 2)}}\text{ } \\
& \Rightarrow \text{ }\!\!\mu\!\!\text{ = }\sqrt{\text{ 3}}\text{ } \\
& \therefore \text{ }\!\!\mu\!\!\text{ = 1}\text{.732 B}\text{.M}\text{.} \\
\end{align}$
Therefore, the magnetic moment of $\text{ C}{{\text{e}}^{\text{3+}}}\text{ }$ ion is equal to $\text{1}\text{.732 B}\text{.M}\text{.}$
So, the correct answer is “Option D”.
Note: Note that, there is a difference in the magnetic moment of the d and f block element. In the case of transition (d) metals the magnetic moment corresponds to the spin only. The magnetic moment arises due to the spin motion of an electron and the orbital motion of an electron. However, in case of d-orbitals are not well shielded from the environment. As a result, the d-electrons of the metal interact strongly with the surrounding ligands. Due to this the orbital motion is quenched and thus the magnetic moment of d-block elements arises only due to the spin motion of the electrons only.
In lanthanides, the orbital motion is not quenched. Thus the effective magnetic moment arises due to spin and orbital motion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




