
The increasing order of $\text{ p}{{\text{K}}_{\text{b}}}\text{ }$ of the following compound is:
A)
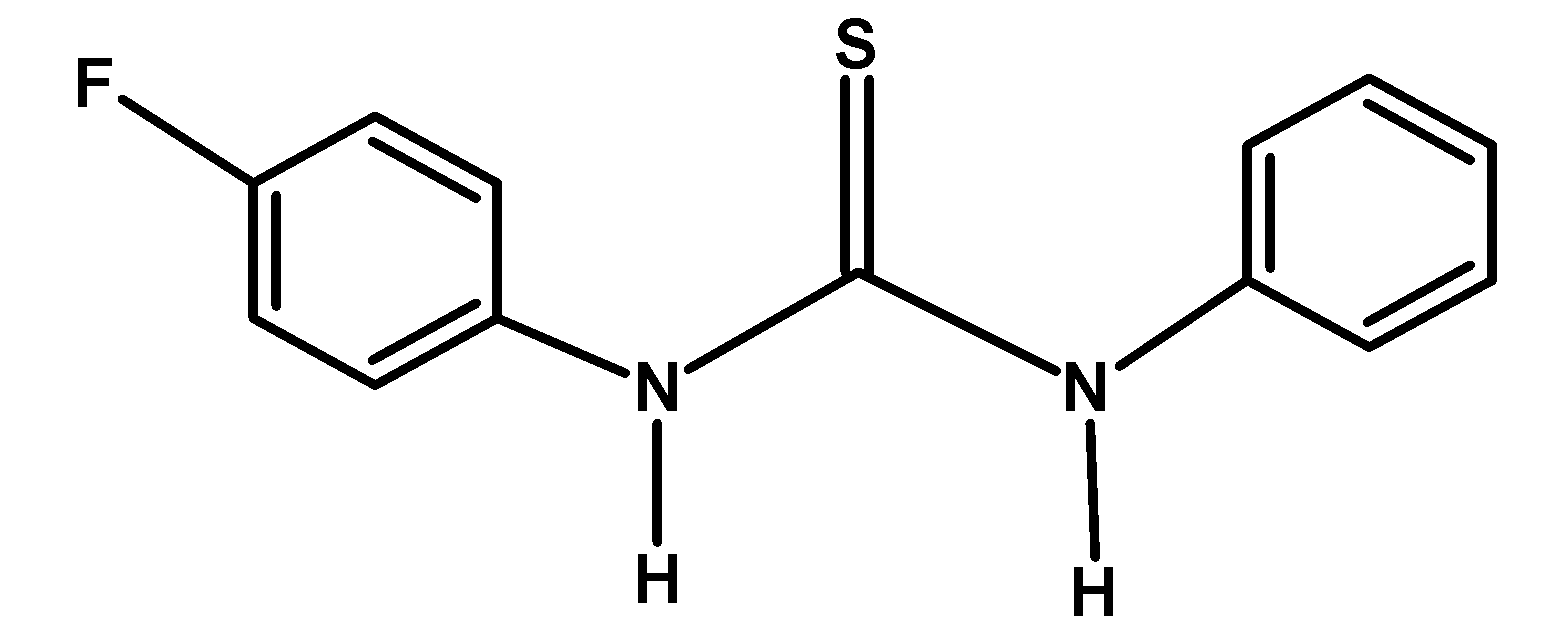
B)

C)
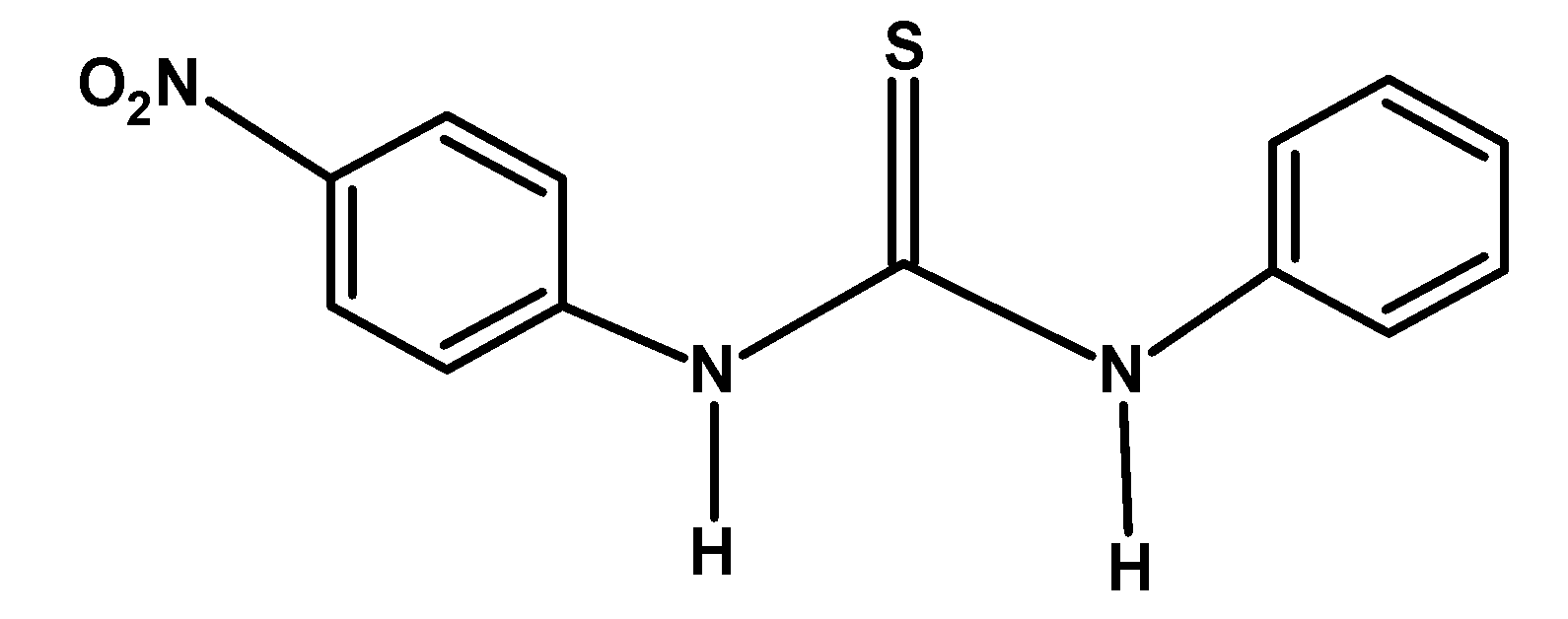
D)
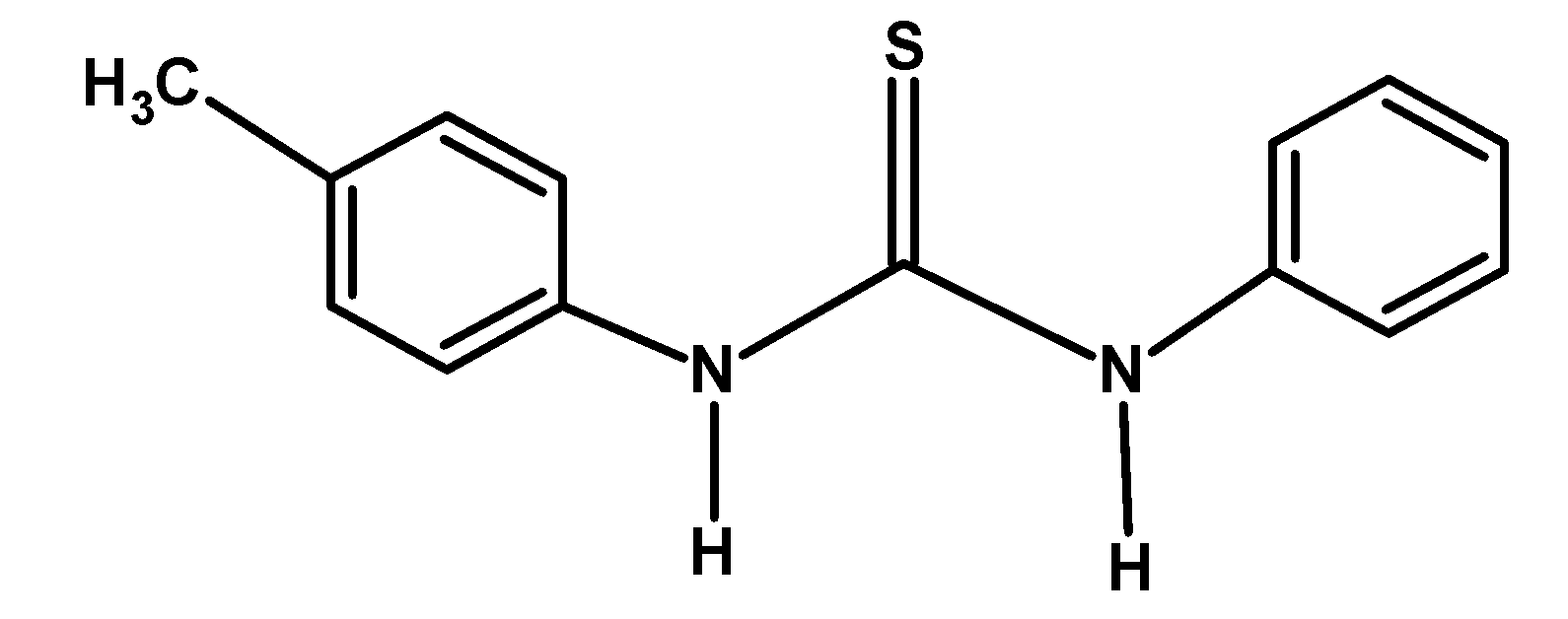
A) $\text{ A }<\text{ C }<\text{ D }<\text{ B }$
B) $\text{ B }<\text{ D }<\text{ A }<\text{ C }$
C) $\text{ C }<\text{ A }<\text{ D }<\text{ B }$
D) $\text{ B }<\text{ D }<\text{ C }<\text{ A }$
| A) | 
|
| B) | 
|
| C) | 
|
| D) | 
|
Answer
577.8k+ views
Hint:. The basicity is the measure of the extent of the base to accept the proton or donate the electron pair. The basicity is expressed as a dissociation constant of the base $\text{ }{{\text{K}}_{\text{b}}}$ . The basicity of an organic compound depends on the substituents. The electron releasing group increases the basicity of the base while the electron-withdrawing group decreases the basicity of the compound.
Complete step by step answer:
The basicity is determined by the $\text{ }{{\text{K}}_{\text{b}}}$ value of a base. the base tends to abstract a proton or donate an electron pair. The value of the $\text{ }{{\text{K}}_{\text{b}}}$ is equilibrium constant for the dissociation of the base.
The basicity depends on the substituents present in the compound.
The electron-donating group likes $-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ and $-\text{OC}{{\text{H}}_{\text{3}}}\text{ }$releases the electron density towards the nitrogen of the compound. This stabilized the cation formed after donating an electron pair or accepting a proton. Thus from given compounds, compound B) and D) have high basicity.
Among $-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ and $-\text{OC}{{\text{H}}_{\text{3}}}\text{ }$ groups, the methyl group donates its electron via the hyperconjugation and inductive effect. However, the methoxy $-\text{OC}{{\text{H}}_{\text{3}}}\text{ }$group donates the electron via the resonance. Thus compound B) has more basicity compared to D).
The electron-withdrawing group like $-\text{F }$ and $-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ withdraws the electron density towards itself from the nitrogen of the compound. This destabilized the cation formed after donating an electron pair or accepting a proton. Thus from given compounds, compound A) and C) have low basicity.
Among $-\text{F }$ and $-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ groups, the Florine group withdraws its electron via the inductive effect. However, the nitro$-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ group withdraws the electron via the resonance. Thus compound A) has more basicity compared to C).
Thus the order of basicity for the compounds is given as,
$\text{ B }>\text{ D }>\text{ A }>\text{ C }$
The $\text{ }{{\text{K}}_{\text{b}}}$ is related to $\text{ p}{{\text{K}}_{\text{b}}}\text{ }$ is given as,’
$\text{ p}{{\text{K}}_{\text{b}}}\text{ }=\text{ }-\log {{\text{K}}_{\text{b}}}\text{ }$
Thus, the order of increasing $\text{ p}{{\text{K}}_{\text{b}}}\text{ }$ is given as,
$\text{ B }<\text{ D }<\text{ A }<\text{ C }$
So, the correct answer is “Option C”.
Note: Note that, we can summarise the basicity and $\text{ p}{{\text{K}}_{\text{b}}}\text{ }$as basicity is directly proportional to the resonance effect of electron releasing group and hyperconjugation effect of electron-donating group. However, the basicity is inversely related to the resonance effect of the electron-withdrawing group and hyperconjugation effect. Always remember that the resonance effect is the permanent effect but hyperconjugation is a temporary effect, thus resonance makes it more stable.
$\text{ Basicity }\propto \text{ +R }\propto \text{ }\dfrac{1}{-\text{R}}\propto \text{ +H }\propto \text{ }\dfrac{1}{-\text{H}}\text{ }$
Complete step by step answer:
The basicity is determined by the $\text{ }{{\text{K}}_{\text{b}}}$ value of a base. the base tends to abstract a proton or donate an electron pair. The value of the $\text{ }{{\text{K}}_{\text{b}}}$ is equilibrium constant for the dissociation of the base.
The basicity depends on the substituents present in the compound.
The electron-donating group likes $-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ and $-\text{OC}{{\text{H}}_{\text{3}}}\text{ }$releases the electron density towards the nitrogen of the compound. This stabilized the cation formed after donating an electron pair or accepting a proton. Thus from given compounds, compound B) and D) have high basicity.
Among $-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ and $-\text{OC}{{\text{H}}_{\text{3}}}\text{ }$ groups, the methyl group donates its electron via the hyperconjugation and inductive effect. However, the methoxy $-\text{OC}{{\text{H}}_{\text{3}}}\text{ }$group donates the electron via the resonance. Thus compound B) has more basicity compared to D).
The electron-withdrawing group like $-\text{F }$ and $-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ withdraws the electron density towards itself from the nitrogen of the compound. This destabilized the cation formed after donating an electron pair or accepting a proton. Thus from given compounds, compound A) and C) have low basicity.
Among $-\text{F }$ and $-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ groups, the Florine group withdraws its electron via the inductive effect. However, the nitro$-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ group withdraws the electron via the resonance. Thus compound A) has more basicity compared to C).
Thus the order of basicity for the compounds is given as,
$\text{ B }>\text{ D }>\text{ A }>\text{ C }$
The $\text{ }{{\text{K}}_{\text{b}}}$ is related to $\text{ p}{{\text{K}}_{\text{b}}}\text{ }$ is given as,’
$\text{ p}{{\text{K}}_{\text{b}}}\text{ }=\text{ }-\log {{\text{K}}_{\text{b}}}\text{ }$
Thus, the order of increasing $\text{ p}{{\text{K}}_{\text{b}}}\text{ }$ is given as,
$\text{ B }<\text{ D }<\text{ A }<\text{ C }$
So, the correct answer is “Option C”.
Note: Note that, we can summarise the basicity and $\text{ p}{{\text{K}}_{\text{b}}}\text{ }$as basicity is directly proportional to the resonance effect of electron releasing group and hyperconjugation effect of electron-donating group. However, the basicity is inversely related to the resonance effect of the electron-withdrawing group and hyperconjugation effect. Always remember that the resonance effect is the permanent effect but hyperconjugation is a temporary effect, thus resonance makes it more stable.
$\text{ Basicity }\propto \text{ +R }\propto \text{ }\dfrac{1}{-\text{R}}\propto \text{ +H }\propto \text{ }\dfrac{1}{-\text{H}}\text{ }$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




