
The increasing order of basicity of the compounds is:
(a)
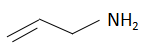
(b)
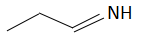
(c)
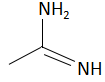
(d)
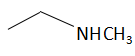
A. (b) < (a) < (c) < (d)
B. (b) < (a) < (d) < (c)
C. (d) < (b) < (a) < (c)
D. (a) < (b) < (c) < (d)




Answer
577.5k+ views
Hint: The basic nature of any compound depends upon the electron donating tendency of the atom having a lone pair of electrons. The basic nature is also affected by certain other factors such as electronegativity, presence of electron donating or electron withdrawing groups around the atom, etc.
Complete step by step answer:
The order of basicity or basic nature depends on how easily an atom can donate the lone pair of electrons or loses the electrons or in simple terms, the electron donating tendency of the atom. In compound (b) the nitrogen atom is \[s{p^2}\] hybridized, so it is the least basic among all the given compounds. The\[s{p^2}\] hybridized carbon has a greater electronegativity and electrons present on an electronegative atom are not easy to donate. Compound (c) is a very strong nitrogenous organic base as the lone pair of electrons on one nitrogen atom delocalized with the help of resonance. This makes another nitrogen atom negatively charged and the resultant conjugate acids have two equivalent resonating structures. Thus, it is most basic in the given compound. Compound (d) is a secondary amine and is more basic than the compound (a) which is a primary amine.
Therefore, the increasing order of basicity is: B. (b) < (a) < (d) < (c)
So, the correct answer is Option B.
Note:
The greater the availability of electrons on the atom, the more is the tendency of the atom to donate it. An electron withdrawing group decreases the energy density on the atoms and an electron donating group increases the electronic density over the atoms. Thus, the electron donating tendency will be:
Tertiary amine > Secondary amine > Primary amine
Complete step by step answer:
The order of basicity or basic nature depends on how easily an atom can donate the lone pair of electrons or loses the electrons or in simple terms, the electron donating tendency of the atom. In compound (b) the nitrogen atom is \[s{p^2}\] hybridized, so it is the least basic among all the given compounds. The\[s{p^2}\] hybridized carbon has a greater electronegativity and electrons present on an electronegative atom are not easy to donate. Compound (c) is a very strong nitrogenous organic base as the lone pair of electrons on one nitrogen atom delocalized with the help of resonance. This makes another nitrogen atom negatively charged and the resultant conjugate acids have two equivalent resonating structures. Thus, it is most basic in the given compound. Compound (d) is a secondary amine and is more basic than the compound (a) which is a primary amine.
Therefore, the increasing order of basicity is: B. (b) < (a) < (d) < (c)
So, the correct answer is Option B.
Note:
The greater the availability of electrons on the atom, the more is the tendency of the atom to donate it. An electron withdrawing group decreases the energy density on the atoms and an electron donating group increases the electronic density over the atoms. Thus, the electron donating tendency will be:
Tertiary amine > Secondary amine > Primary amine
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




