
The hybridization of carbon in diamond, graphite and acetylene is:
\[
A.\;\;\;\;\;s{p^3},{\text{ }}s{p^2},{\text{ }}sp \\
B.\;\;\;\;\;s{p^3},{\text{ }}sp,{\text{ }}s{p^2} \\
C.\;\;\;\;\;s{p^2},{\text{ }}s{p^3},{\text{ }}sp \\
D.\;\;\;\;\;sp,{\text{ }}s{p^3},{\text{ }}s{p^2} \\
\]
Answer
594.9k+ views
Hint: We have to find the hybridization of carbon in diamond, graphite, and acetylene. Therefore, we must imagine the structure and types of bonds present in these compounds. And also we must understand the different allotropic nature of carbon. Then only we can find the hybridization of carbon in different compounds.
Complete step by step solution:
We know that the carbon exists in various allotropic states (the phenomenon of presence of an element in 2 or more than 2 forms with different atom arrangements in crystalline solid form or in molecule occurrence that contain atoms of a different number). Out of these allotropes, 2 are diamond and graphite.
Diamond is known for its perfect tetrahedron structure throughout the crystal with all the carbon atoms strongly bonded by other carbon atoms in the crystal with 4 sigma bonds each (i.e. all the valences of the carbon are fulfilled), thus giving rise to $sp^3$ hybridization.
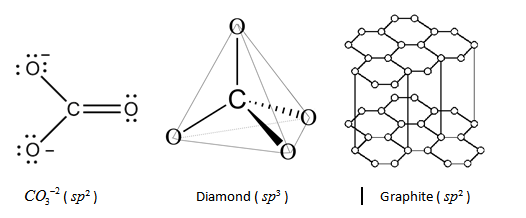
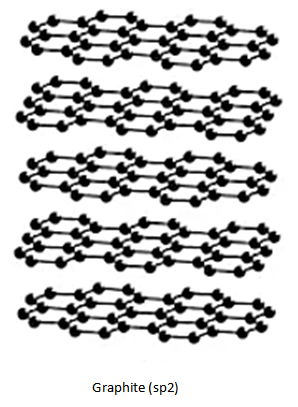
Graphite, on the other hand, makes sheets like structure very similar to chicken wire. Carbon atoms in graphite are chemically bonded with the other three carbon atoms (1 free valence is present), in the same plane giving rise to a hexagonal ring which in turn produces $sp^2$ hybridization.
In acetylene, the carbon to carbon bond is formed by three bonds namely, 1 sigma bond and 2 pi bonds. This type of chemical hybridization in a compound is termed as $sp$ type hybridization.
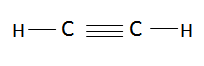
So the carbon hybridization in diamond, graphite, and acetylene are \[s{p^3}\] hybridization, \[s{p^2}\] hybridization, and \[sp\] hybridization respectively as proved with the above reasons.
And hence, option A is correct.
Note: We must understand that the diamond is the hardest substance known on earth due to its tetrahedron structure. In graphite due to the presence of delocalized electrons, it becomes a good conductor of electricity.
Complete step by step solution:
We know that the carbon exists in various allotropic states (the phenomenon of presence of an element in 2 or more than 2 forms with different atom arrangements in crystalline solid form or in molecule occurrence that contain atoms of a different number). Out of these allotropes, 2 are diamond and graphite.
Diamond is known for its perfect tetrahedron structure throughout the crystal with all the carbon atoms strongly bonded by other carbon atoms in the crystal with 4 sigma bonds each (i.e. all the valences of the carbon are fulfilled), thus giving rise to $sp^3$ hybridization.

Graphite, on the other hand, makes sheets like structure very similar to chicken wire. Carbon atoms in graphite are chemically bonded with the other three carbon atoms (1 free valence is present), in the same plane giving rise to a hexagonal ring which in turn produces $sp^2$ hybridization.

In acetylene, the carbon to carbon bond is formed by three bonds namely, 1 sigma bond and 2 pi bonds. This type of chemical hybridization in a compound is termed as $sp$ type hybridization.
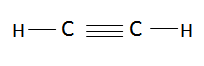
So the carbon hybridization in diamond, graphite, and acetylene are \[s{p^3}\] hybridization, \[s{p^2}\] hybridization, and \[sp\] hybridization respectively as proved with the above reasons.
And hence, option A is correct.
Note: We must understand that the diamond is the hardest substance known on earth due to its tetrahedron structure. In graphite due to the presence of delocalized electrons, it becomes a good conductor of electricity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




