
The hybridisation of nitrogen atom in pyridine is:
(A) $\text{s}{{\text{p}}^{3}}$
(B) $\text{s}{{\text{p}}^{2}}$
(C) $\text{sp}$
(D) $\text{s}{{\text{p}}^{3}}\text{d}$
Answer
595.5k+ views
Hint: Draw the structure of pyridine. Identify the number of bonds and type of bonds($\sigma ,\pi $) present. Hybridisation of an organic compound is given by the VBT. Valence Bond theory decides hybridisation based on the number of $\sigma $bonds and lone pair of electrons attached to the atom of consideration.
Complete step by step answer:
According to valence bond theory, electrons in a molecule occupy atomic orbitals and not molecular orbitals. The atomic orbitals overlap on the bond formation and the strength of the bond depends on the extent of overlap.
Postulates of Valence Bond Theory:
- Covalent bonds are formed when two valence orbitals belonging to two different atoms overlap onto each other. Due to overlapping the electron density in that region increases, thereby increasing the stability of the molecule thus formed.
- The presence of many unpaired electrons in the valence shell of an atom enables the atoms to form multiple bonds with each other. However, the paired electrons present in the valent shell do not take part in formation of chemical bonds.
- Covalent bonds are directional and parallel to the region corresponding to atomic orbitals that are going to overlap.
- Sigma bonds and pi bonds differ in the pattern that the atomic orbitals overlap in, i.e. sigma bonds undergo head on overlap however pi bonds undergo sideways overlapping.
For calculation of hybridization we consider only sigma bonds and lone pairs of electrons and not the pi bonds. Let us draw the structure of pyridine and identify the types of bonds present around nitrogen atom:
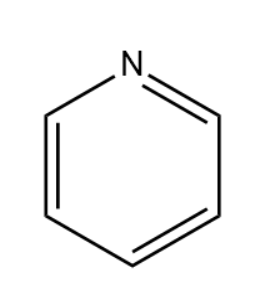
Number of sigma bonds = 2
Number of lone pairs = 1
Coordination number = 2+1 = 3
For coordination number 3, the hybridisation is $\text{s}{{\text{p}}^{2}}$.
So, the correct answer is “Option B”.
Note: Valence bond theory was successful in determining the hybridization of atoms in a molecule. However, the theory had some limitations ,like:
- Unable to explain the tetravalency of carbon.
- No insight or information on the energies of electrons.
- Incorrect assumption that electrons are localized in specific areas only,
- No distinction between weak and strong ligands ( hybridization of complex compounds).
Complete step by step answer:
According to valence bond theory, electrons in a molecule occupy atomic orbitals and not molecular orbitals. The atomic orbitals overlap on the bond formation and the strength of the bond depends on the extent of overlap.
Postulates of Valence Bond Theory:
- Covalent bonds are formed when two valence orbitals belonging to two different atoms overlap onto each other. Due to overlapping the electron density in that region increases, thereby increasing the stability of the molecule thus formed.
- The presence of many unpaired electrons in the valence shell of an atom enables the atoms to form multiple bonds with each other. However, the paired electrons present in the valent shell do not take part in formation of chemical bonds.
- Covalent bonds are directional and parallel to the region corresponding to atomic orbitals that are going to overlap.
- Sigma bonds and pi bonds differ in the pattern that the atomic orbitals overlap in, i.e. sigma bonds undergo head on overlap however pi bonds undergo sideways overlapping.
For calculation of hybridization we consider only sigma bonds and lone pairs of electrons and not the pi bonds. Let us draw the structure of pyridine and identify the types of bonds present around nitrogen atom:

Number of sigma bonds = 2
Number of lone pairs = 1
Coordination number = 2+1 = 3
For coordination number 3, the hybridisation is $\text{s}{{\text{p}}^{2}}$.
So, the correct answer is “Option B”.
Note: Valence bond theory was successful in determining the hybridization of atoms in a molecule. However, the theory had some limitations ,like:
- Unable to explain the tetravalency of carbon.
- No insight or information on the energies of electrons.
- Incorrect assumption that electrons are localized in specific areas only,
- No distinction between weak and strong ligands ( hybridization of complex compounds).
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Explain zero factorial class 11 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

State and prove Bernoullis theorem class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




