
The formula of Aluminium oxide is \[A{{l}_{2}}{{O}_{3}}\]. Find the valencies of Aluminium and Oxygen.
Answer
617.7k+ views
- Hint: We know that the atomic number of Aluminium is 13 and the atomic number of Oxygen is 8. Using the criss-cross method we can determine the valency of Aluminium and Oxygen in Aluminium oxide or \[A{{l}_{2}}{{O}_{3}}\].
Complete step by step answer:
We know that according to Bohr’s atomic model, the number of electrons that can be present on the different shells is determined by the formula \[2({{n}^{2}})\], where n denotes the number of the shell.
Since the atomic number of Aluminium is 13, using the formula \[2({{n}^{2}})\] we get,
In the first shell, the number of electrons is \[2\times {{1}^{2}}=2\] electrons.
In the second shell, the number of electrons is \[2\times {{2}^{2}}=8\] electrons.
In the third shell, the number of valence electrons is = 3 electrons.
Since \[2+8+3=13\] electrons, the atomic number of Aluminium is 13.
Now, the atomic number of Oxygen is 8. Using the formula \[2({{n}^{2}})\] we get,
In the first shell, the number of electrons is \[2\times {{1}^{2}}=2\] electrons
In the second shell, the number of valence electrons = 6 electrons.
Since \[2+6=8\] electrons, the atomic number of Oxygen is 8.
Therefore, to attain the octet configuration, Aluminium loses 3 electrons, and Oxygen gains 2 electrons.
Now, we have to write the formula of Aluminium Oxide using the criss-cross method.
We know that in the criss-cross method the numerical value of each of the ion charges is crossed over to become the subscript of the other ion.
The charge of Aluminium is +3 and the charge of Oxygen is -2.
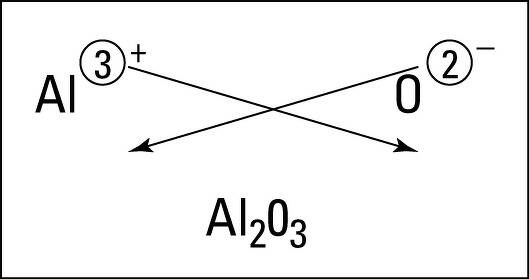
Therefore, we can conclude that the valency of Aluminium in \[A{{l}_{2}}{{O}_{3}}\] is 3 and the valency of Oxygen in \[A{{l}_{2}}{{O}_{3}}\] is 2.
Note: Aluminium loses the 3 valence electrons to gain the noble gas configuration of Neon, which is 2,8.
Similarly, Oxygen gains 2 valence electrons to gain the noble gas configuration of Neon, that is 2,8.
Complete step by step answer:
We know that according to Bohr’s atomic model, the number of electrons that can be present on the different shells is determined by the formula \[2({{n}^{2}})\], where n denotes the number of the shell.
Since the atomic number of Aluminium is 13, using the formula \[2({{n}^{2}})\] we get,
In the first shell, the number of electrons is \[2\times {{1}^{2}}=2\] electrons.
In the second shell, the number of electrons is \[2\times {{2}^{2}}=8\] electrons.
In the third shell, the number of valence electrons is = 3 electrons.
Since \[2+8+3=13\] electrons, the atomic number of Aluminium is 13.
Now, the atomic number of Oxygen is 8. Using the formula \[2({{n}^{2}})\] we get,
In the first shell, the number of electrons is \[2\times {{1}^{2}}=2\] electrons
In the second shell, the number of valence electrons = 6 electrons.
Since \[2+6=8\] electrons, the atomic number of Oxygen is 8.
Therefore, to attain the octet configuration, Aluminium loses 3 electrons, and Oxygen gains 2 electrons.
Now, we have to write the formula of Aluminium Oxide using the criss-cross method.
We know that in the criss-cross method the numerical value of each of the ion charges is crossed over to become the subscript of the other ion.
The charge of Aluminium is +3 and the charge of Oxygen is -2.

Therefore, we can conclude that the valency of Aluminium in \[A{{l}_{2}}{{O}_{3}}\] is 3 and the valency of Oxygen in \[A{{l}_{2}}{{O}_{3}}\] is 2.
Note: Aluminium loses the 3 valence electrons to gain the noble gas configuration of Neon, which is 2,8.
Similarly, Oxygen gains 2 valence electrons to gain the noble gas configuration of Neon, that is 2,8.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




