
The formula for ammonia is $N{H_3}$ .
A.True
B.False
Answer
581.1k+ views
Hint: The chemical formula of Ammonia is $N{H_3}$ . Ammonia consists of Hydrogen and Nitrogen. It is also known as trihydride nitrogen and also nitrogen trihydride. The density of Ammonia is 0.769 $\dfrac{{kg}}{{{m^3}}}$ at STP and it is lighter than air.
Complete step by step answer:
Ammonia is made of Nitrogen and Hydrogen. The electron configuration of Nitrogen (N) is \[1{s^2}2{s^2}2{p^3}\] . We can see that it needs 3 electrons to complete stability. The electronic configuration of Hydrogen (H) is \[1{s^2}\]
All the three hydrogen share their 1 electron with the Nitrogen and in this way, Nitrogen gains stability.
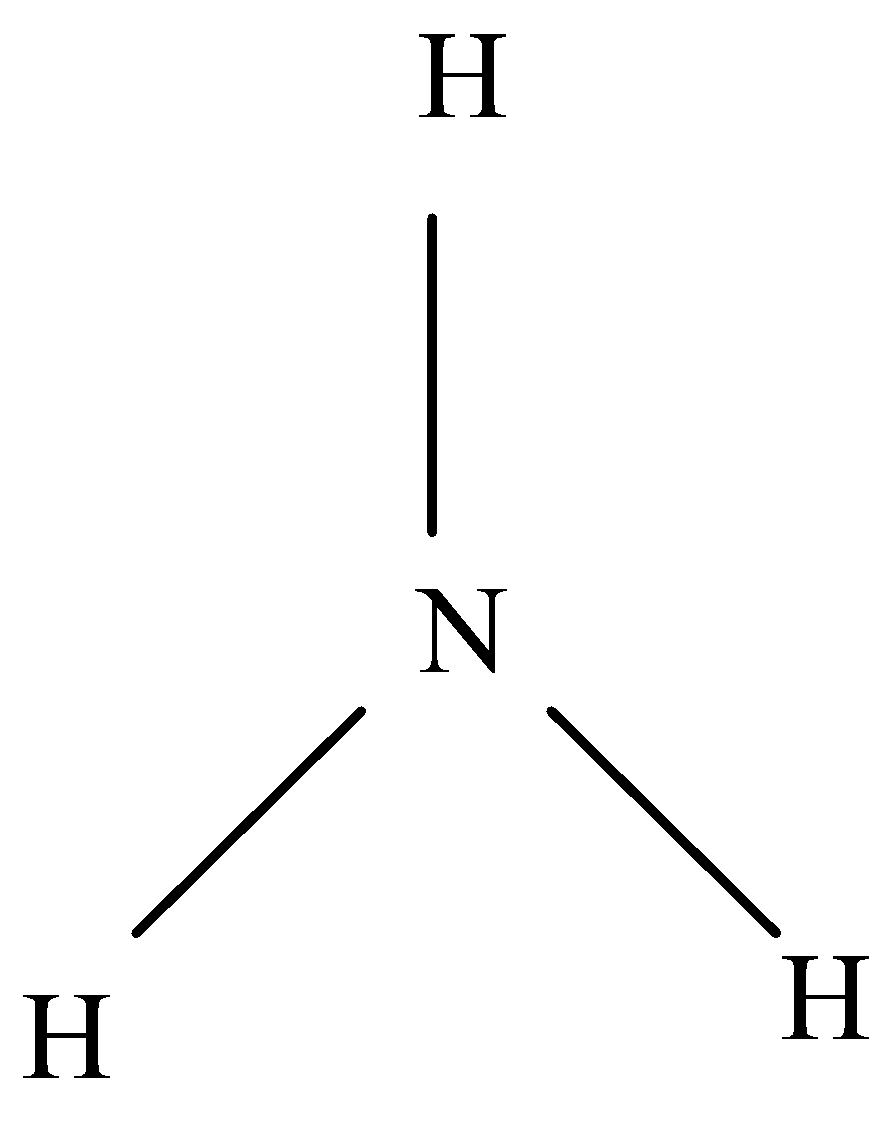
Ammonia is made in the laboratory by heating ammonium salt such as ammonium chloride with a strong alkali such as calcium hydroxide or sodium hydroxide. This process is known as Haber process, the combination of nitrogen and hydrogen under high pressure in the presence of a catalyst.
So, the formula of Ammonia is $N{H_3}$ .
Therefore, the correct answer is option (A).
Note: Ammonia behaves as a weak base because it combines with most acids to form salts. When Ammonia reacts with Hydrochloric acid, it converts into ammonium chloride. Ammonia is used in a household as the cleaner, it is mixed with water to clean the steel and glass, to increase the yield of crops, it is used as fertilizers, is used as a refrigerant, is used in the fermentation industry, is used in the fuels for rocket engine and is a preferred nitrogen containing nutrient for the growth of plants. Ammonia occurs naturally in soil, air or in vegetation. Ammonia is also a common pollutant and can be toxic and cause lower reproduction or even death.
Complete step by step answer:
Ammonia is made of Nitrogen and Hydrogen. The electron configuration of Nitrogen (N) is \[1{s^2}2{s^2}2{p^3}\] . We can see that it needs 3 electrons to complete stability. The electronic configuration of Hydrogen (H) is \[1{s^2}\]
All the three hydrogen share their 1 electron with the Nitrogen and in this way, Nitrogen gains stability.

Ammonia
Ammonia is made in the laboratory by heating ammonium salt such as ammonium chloride with a strong alkali such as calcium hydroxide or sodium hydroxide. This process is known as Haber process, the combination of nitrogen and hydrogen under high pressure in the presence of a catalyst.
So, the formula of Ammonia is $N{H_3}$ .
Therefore, the correct answer is option (A).
Note: Ammonia behaves as a weak base because it combines with most acids to form salts. When Ammonia reacts with Hydrochloric acid, it converts into ammonium chloride. Ammonia is used in a household as the cleaner, it is mixed with water to clean the steel and glass, to increase the yield of crops, it is used as fertilizers, is used as a refrigerant, is used in the fermentation industry, is used in the fuels for rocket engine and is a preferred nitrogen containing nutrient for the growth of plants. Ammonia occurs naturally in soil, air or in vegetation. Ammonia is also a common pollutant and can be toxic and cause lower reproduction or even death.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




