
The following reaction is suppose to take place through ${S_{{N^1}}}$ mechanism
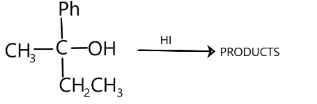
If the configuration of substrate is D, then the configuration of products will be:
I) D
II) L
III) $50\% D\& 50\% L$
IV) May be D or L

Answer
547.5k+ views
Hint:As we know that nucleophilic substitution reaction are of two types: (1) ${S_{{N^1}}}$ reaction (2)${S_{{N^2}}}$reaction. Nucleophilic substitution reactions are such reactions in which stronger nucleophiles displaces the weaker nucleophile. First, we will find out the products via ${S_{{N^1}}}$ mechanism. After that using rules of D and L configuration, we will find out the configuration of the product molecule.
Complete step-by-step answer:In alcohols, the C site is susceptible to attack by nucleophiles (an electron rich species). Thus, when a nucleophile approaches the positively charged carbon, the bond between the alcoholic group and carbon atom will break and new bond will form between halogen and the carbon and forms the alkyl halides. This reaction proceeds via ${S_{{N^1}}}$ mechanism. ${S_{{N^1}}}$ mechanism proceeds via formation of carbonium ion. It is ionization step. It is rate determining step too. In the second step nucleophile attacks the carbocation to give the substituted product immediately as carbocation is highly reactive species. This step is fast step hence it does not affect the rate of reaction. It can be illustrated as:
$ROH + HX \to RX + {H_2}O$
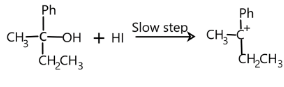
Let us treat our reactant in the same way. First, there will be formation of carbonium ions. It is a slow step.
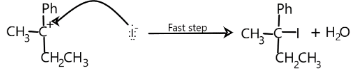
In the second step,$HI$ acts as a nucleophile and attacks the carbocation and forms the product. The carbocation is unstable. To achieve stability it will react with iodine and forms the alkyl halide and there is elimination of water.
There are some rules to write the configuration. The rules are followed as: (1) if the rotation of the plane is to the right (clockwise), the substance is dextrorotatory. On the other hand, if the rotation of the plane is to the left (anticlockwise), the substance is laevorotatory. You can say that if the $OH$group is attached to the carbon in the right side, the molecule has a D configuration and if it is attached to the left side then the molecule has an L configuration. In our case OH group is replaced by iodine and it is on the right side. Therefore, it also has D configuration.
So, option (A) is correct.
Note:Remember that strong nucleophiles can displace weak nucleophiles. Among the hydrogen halides the decreasing order of reactivity is:$HI > HBr > HCl$. Keep in mind that there is a difference between relative and absolute configuration.
Complete step-by-step answer:In alcohols, the C site is susceptible to attack by nucleophiles (an electron rich species). Thus, when a nucleophile approaches the positively charged carbon, the bond between the alcoholic group and carbon atom will break and new bond will form between halogen and the carbon and forms the alkyl halides. This reaction proceeds via ${S_{{N^1}}}$ mechanism. ${S_{{N^1}}}$ mechanism proceeds via formation of carbonium ion. It is ionization step. It is rate determining step too. In the second step nucleophile attacks the carbocation to give the substituted product immediately as carbocation is highly reactive species. This step is fast step hence it does not affect the rate of reaction. It can be illustrated as:
$ROH + HX \to RX + {H_2}O$
Let us treat our reactant in the same way. First, there will be formation of carbonium ions. It is a slow step.

In the second step,$HI$ acts as a nucleophile and attacks the carbocation and forms the product. The carbocation is unstable. To achieve stability it will react with iodine and forms the alkyl halide and there is elimination of water.

There are some rules to write the configuration. The rules are followed as: (1) if the rotation of the plane is to the right (clockwise), the substance is dextrorotatory. On the other hand, if the rotation of the plane is to the left (anticlockwise), the substance is laevorotatory. You can say that if the $OH$group is attached to the carbon in the right side, the molecule has a D configuration and if it is attached to the left side then the molecule has an L configuration. In our case OH group is replaced by iodine and it is on the right side. Therefore, it also has D configuration.
So, option (A) is correct.
Note:Remember that strong nucleophiles can displace weak nucleophiles. Among the hydrogen halides the decreasing order of reactivity is:$HI > HBr > HCl$. Keep in mind that there is a difference between relative and absolute configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




