
The final product of the given reaction is,
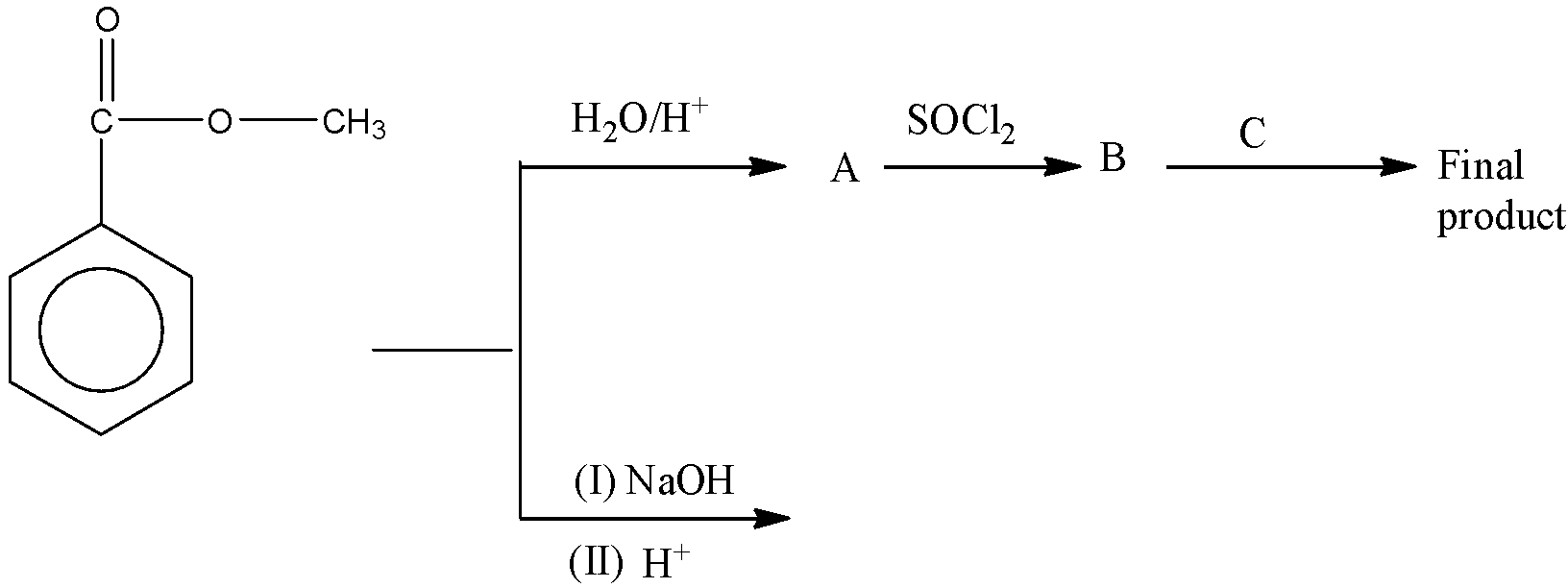

Answer
561k+ views
Hint: Here, first we have to find out the product C of the second reaction. We know that an ester when reacts with sodium hydroxide forms carboxylate ion and alcohol. Further reaction with acid produces the carboxylic acid.
Complete step by step answer:
Let’s try to find the product C in the second reaction. The given reactant $Ph-COOCH_3$ is an ester. And we know the reaction of ester with sodium hydroxide produces carboxylate ion and alcohol. Then, the reaction of carboxylate ion with acid gives carboxylic acid. The reaction can be shown as follows:
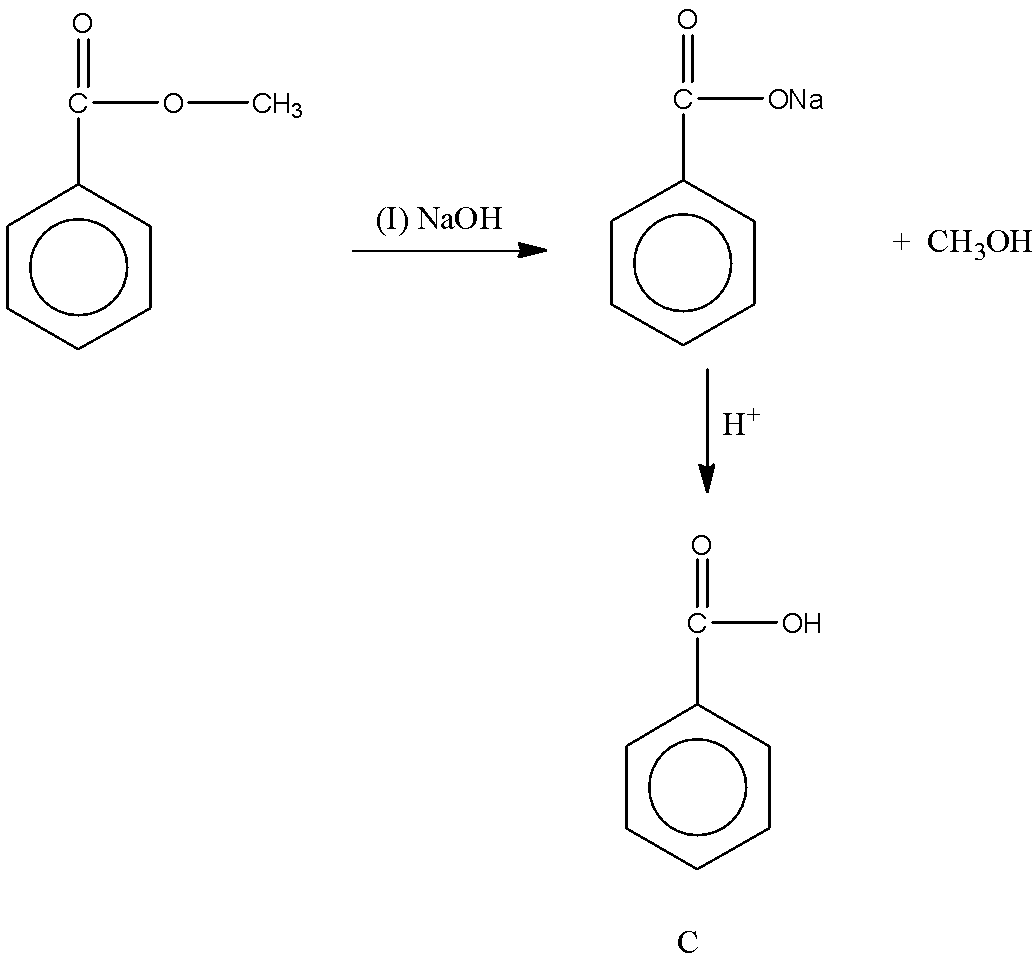
So, we obtain the product C.
Now we have to find the final product of the first reaction.
Here, $Ph-COOCH_3$ reacts with water to produce an acid and an alcohol in presence of an acid.
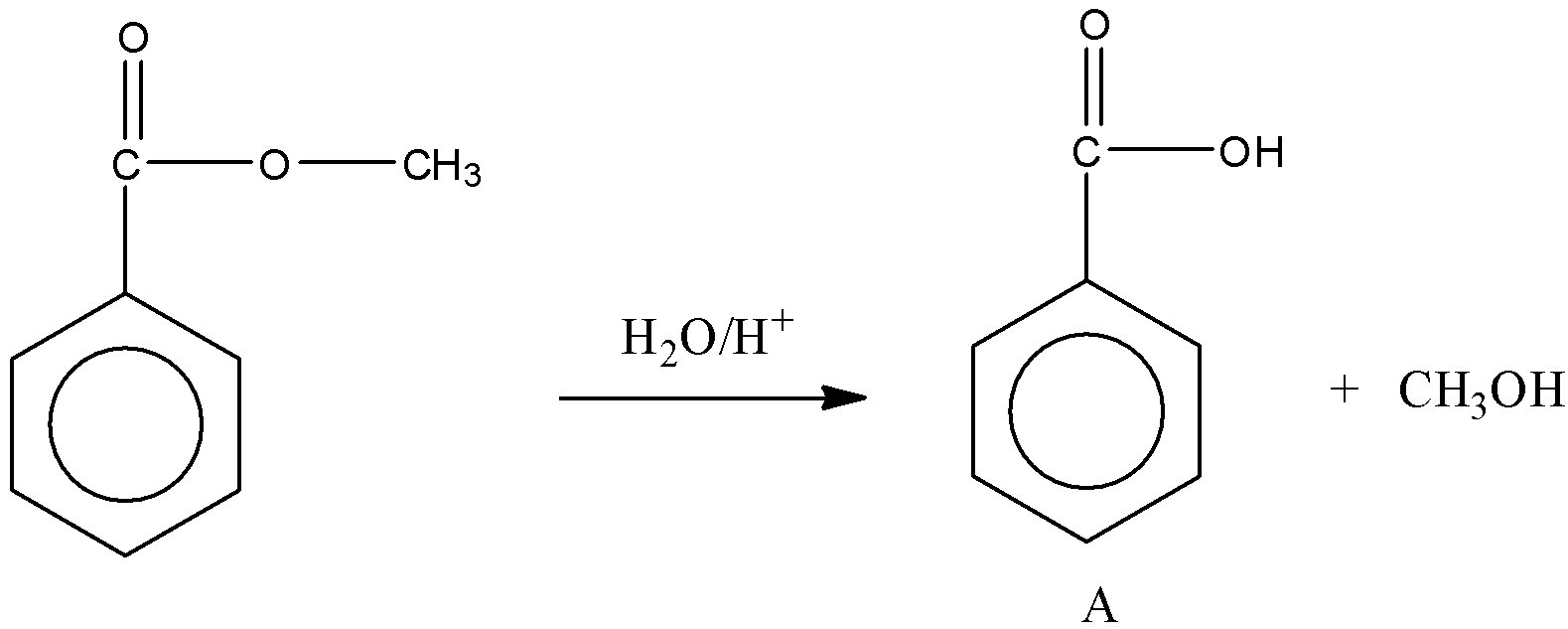
So, we obtain the product A.
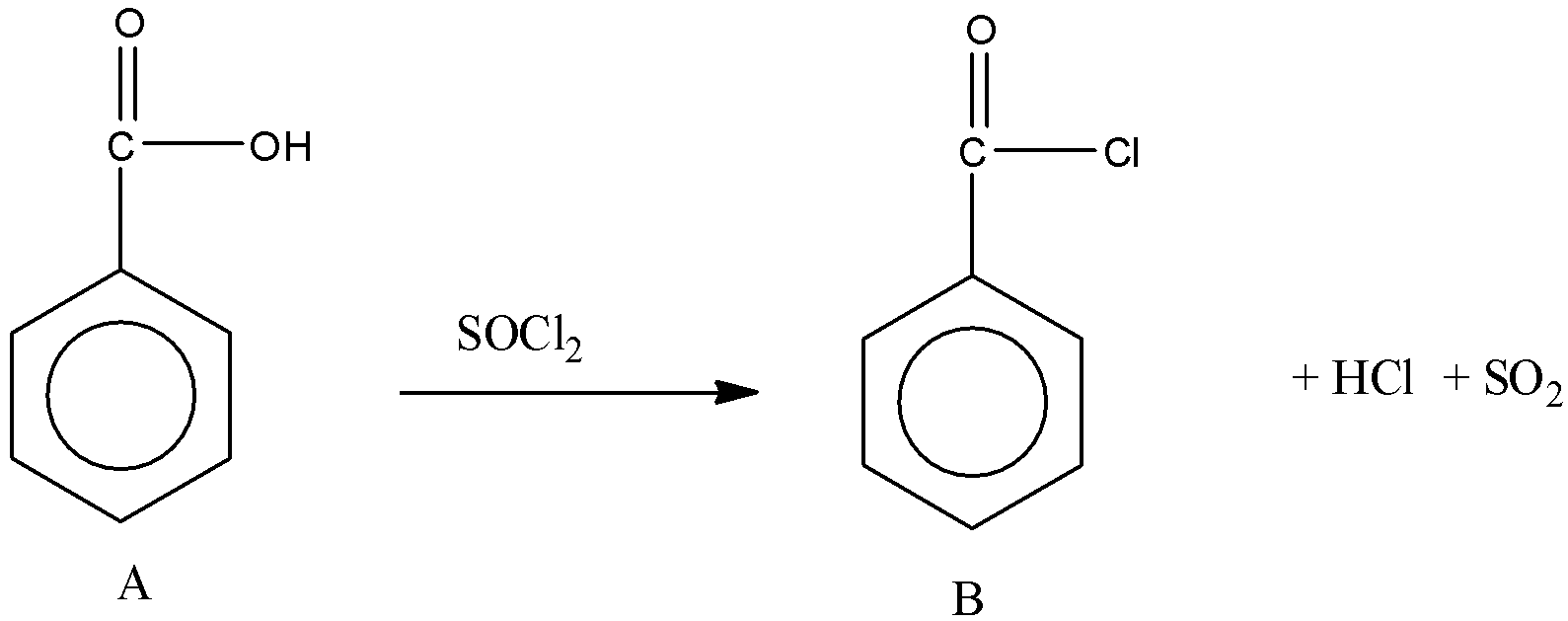
Then, A reacts with ${\rm{SOC}}{{\rm{l}}_{\rm{2}}}$. We know that when carboxylic acid reacts with ${\rm{SOC}}{{\rm{l}}_{\rm{2}}}$, the product formed is acid chloride.
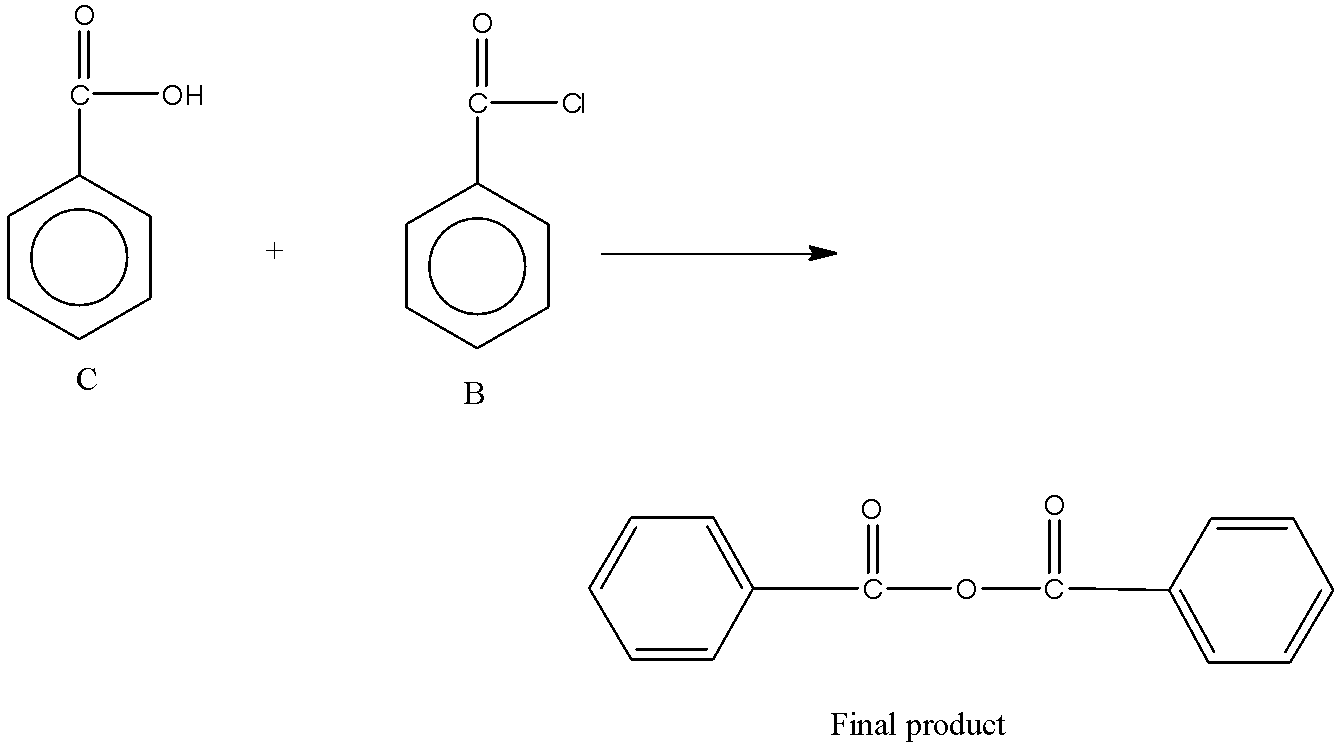
Now, the acid chloride undergoes reaction with C (second reaction). The product C formed is a carboxylic acid. So, the reaction is between acid chloride and carboxylic acid. The reaction between carboxylic acid and acid chloride forms acid anhydride. So, the reaction is,
Note: It is to be noted that the reaction of ester with sodium hydroxide is also known by the name of saponification reaction. In this reaction, fat, lipid or oil is transformed into alcohol and soap by heating it in presence of sodium hydroxide. The saponification value indicates the quantity of base (NaOH) needed to saponify a sample of fat.
Complete step by step answer:
Let’s try to find the product C in the second reaction. The given reactant $Ph-COOCH_3$ is an ester. And we know the reaction of ester with sodium hydroxide produces carboxylate ion and alcohol. Then, the reaction of carboxylate ion with acid gives carboxylic acid. The reaction can be shown as follows:

So, we obtain the product C.
Now we have to find the final product of the first reaction.
Here, $Ph-COOCH_3$ reacts with water to produce an acid and an alcohol in presence of an acid.

So, we obtain the product A.
Then, A reacts with ${\rm{SOC}}{{\rm{l}}_{\rm{2}}}$. We know that when carboxylic acid reacts with ${\rm{SOC}}{{\rm{l}}_{\rm{2}}}$, the product formed is acid chloride.

Now, the acid chloride undergoes reaction with C (second reaction). The product C formed is a carboxylic acid. So, the reaction is between acid chloride and carboxylic acid. The reaction between carboxylic acid and acid chloride forms acid anhydride. So, the reaction is,

Note: It is to be noted that the reaction of ester with sodium hydroxide is also known by the name of saponification reaction. In this reaction, fat, lipid or oil is transformed into alcohol and soap by heating it in presence of sodium hydroxide. The saponification value indicates the quantity of base (NaOH) needed to saponify a sample of fat.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




