
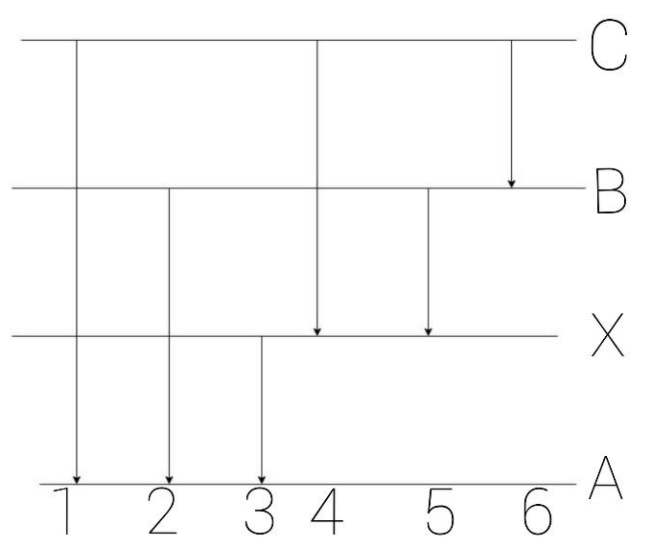
The figure indicates the energy level diagram for the origin of six spectral lines in the emission spectrum (e.g. line no. $5$ arises from the transition from level B to X). Which of the following spectral lines will not occur in the absorption spectrum?
(a) $1,2,3$
(b) $3,2$
(c) $4,5,6$
(d) $3,2,1$

Answer
561k+ views
Hint:For these questions, we have to know which absorption spectrum, what these diagrams show and how we understand this diagram. C, B, X and A are the different energy levels and $1,2,3,4,5$ and $6$ are six different spectral lines.
Complete step by step solution:
Starting with the definition of the Absorption Spectrum,
Absorption Spectrum: Any spectral line which transitions from higher excited state to lower excited state and lower excited state must be ground state and occur in the absorption spectrum.
In these questions, the highest energy level is of C and the ground energy level is as A. Different spectral lines have different higher energy levels, the transition where it starts will be its highest energy level.
Now, thinking about all the spectral lines one-by-one.
- In the spectral line $1$, its transition arises from C to A, so this spectral line transition is from higher excited state to ground excited state. Hence, occur in the absorption spectrum.
- Similarly, in the spectral line $2$, its transition arises from B to A, so, this spectral line transition is from higher excited state to ground excited state. Hence, occur in the absorption spectrum.
- Similarly, in the spectral line $3$, its transition arises from X to A, so, this spectral line transition is from higher excited state to ground excited state. Hence, occur in the absorption spectrum.
- Similarly, in the spectral line $4$, its transition arises from C to B, so this spectral line transition is not from higher excited state to ground excited state. Hence, not occur in the absorption spectrum.
- Similarly, in the spectral line $5$, its transition arises from B to X, so this spectral line transition is not from higher excited state to ground excited state. Hence, not occur in the absorption spectrum.
- Similarly, in the spectral line $6$, its transition arises from C to B, so this spectral line transition is not from higher excited state to ground excited state. Hence, not occur in the absorption spectrum.
So, from the above discussions, the spectral lines which do not occur in the absorption spectrum are $4,5,6$.
Hence, the correct option is (c) $4,5,6$.
Note:The diagram is the main one in this type of question. The spectral line transition arises where the tail of the arrow starts from and ends at the position where the head of the arrow is ended. The absorption spectrum in which the spectral lines arise transition from higher excited state to the ground state.
Complete step by step solution:
Starting with the definition of the Absorption Spectrum,
Absorption Spectrum: Any spectral line which transitions from higher excited state to lower excited state and lower excited state must be ground state and occur in the absorption spectrum.
In these questions, the highest energy level is of C and the ground energy level is as A. Different spectral lines have different higher energy levels, the transition where it starts will be its highest energy level.
Now, thinking about all the spectral lines one-by-one.
- In the spectral line $1$, its transition arises from C to A, so this spectral line transition is from higher excited state to ground excited state. Hence, occur in the absorption spectrum.
- Similarly, in the spectral line $2$, its transition arises from B to A, so, this spectral line transition is from higher excited state to ground excited state. Hence, occur in the absorption spectrum.
- Similarly, in the spectral line $3$, its transition arises from X to A, so, this spectral line transition is from higher excited state to ground excited state. Hence, occur in the absorption spectrum.
- Similarly, in the spectral line $4$, its transition arises from C to B, so this spectral line transition is not from higher excited state to ground excited state. Hence, not occur in the absorption spectrum.
- Similarly, in the spectral line $5$, its transition arises from B to X, so this spectral line transition is not from higher excited state to ground excited state. Hence, not occur in the absorption spectrum.
- Similarly, in the spectral line $6$, its transition arises from C to B, so this spectral line transition is not from higher excited state to ground excited state. Hence, not occur in the absorption spectrum.
So, from the above discussions, the spectral lines which do not occur in the absorption spectrum are $4,5,6$.
Hence, the correct option is (c) $4,5,6$.
Note:The diagram is the main one in this type of question. The spectral line transition arises where the tail of the arrow starts from and ends at the position where the head of the arrow is ended. The absorption spectrum in which the spectral lines arise transition from higher excited state to the ground state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




