
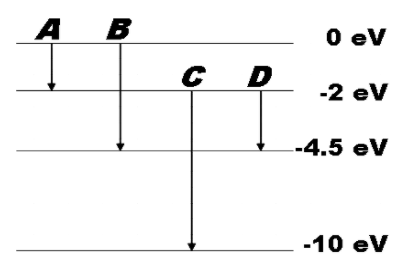
The energy levels of an atom are shown below. Which of them will result in the transition of a photon of wavelength 275nm? Which of these transitions correspond to emissions of radiations of (a) maximum wavelength (b) Minimum wavelength?

Answer
573.9k+ views
Hint: Firstly you could recall the expression for energy in terms of wavelength. Then, using this expression you could find the energy of the photon of given wavelength in $eV$ and then find which among the given transition corresponds to that value. Now using the fact that maximum wavelength is for the minimum emission of energy and minimum wavelength is for maximum emission of energy, find the answer for the subparts.
Formula used:
Energy of emitted radiation,
$E=\dfrac{hc}{\lambda }$
Complete answer:
In the question, we are given the various energy levels of an atom along with transitions A, B, C and D taking place with emission of radiation.
We are asked to identify the transition that corresponds to the transition of a photon of wavelength 275nm and also transitions of minimum and maximum wavelengths.
We know that the energy of a photon is given by,
$E=h\nu $ ……………………………………………. (1)
Where, h is the Planck’s constant given by,
$h=6.63\times {{10}^{-34}}{{m}^{2}}kg/s$
Where, $\nu $ is the frequency of the radiation emitted given by,
$\nu =\dfrac{c}{\lambda }$ ………………………………………………. (2)
Substituting (2) in (1), we get,
$E=\dfrac{hc}{\lambda }$ ……………………. (3)
Where c is the universal speed of light in vacuum given by,
$c=3\times {{10}^{8}}m/s$
Now, by substituting the values we get,
$E=\dfrac{6.63\times {{10}^{-34}}\times 3\times {{10}^{8}}}{275\times {{10}^{-9}}}$
$E=0.072\times {{10}^{-17}}J$ ……………………. (4)
We know that, one electron volt is given by,
$1eV=1.6\times {{10}^{-19}}J$
We could now convert (4) into electron volts.
$E=\dfrac{0.072\times {{10}^{-17}}}{1.6\times {{10}^{-19}}}eV$
$\therefore E=4.5eV$
Energy of emitted radiation is given by the difference of energy of the energy levels across which the transition takes place.
So the transition that corresponds to the transition of a photon of wavelength 275nm will be transition B whose energy of emitted radiation will be the same as that of the photon.
$0-\left( -4.5eV \right)=4.5eV$
From equation (3) we see that energy is inversely proportional to the wavelength of radiation. So the transition that emits radiation of least energy will have the maximum wavelength and the radiation with maximum energy will have minimum wavelength.
(a) Minimum energy emitted will be for transition A,
$0-\left( -2eV \right)=2eV$
So the maximum wavelength will also be for the radiation emitted in transition A.
(b) Maximum energy emitted will be for transition C,
$-2eV-\left( -10eV \right)=8eV$
So the minimum wavelength will also be for the radiation emitted in transition C.
Note:
In case you wonder what these energy levels are, they are the fixed distances from the nucleus of an atom where there is a possibility for the electrons to be found. Energy levels could be compared to steps of a staircase. The electrons can be present in one or the other energy level but not in between.
Formula used:
Energy of emitted radiation,
$E=\dfrac{hc}{\lambda }$
Complete answer:
In the question, we are given the various energy levels of an atom along with transitions A, B, C and D taking place with emission of radiation.
We are asked to identify the transition that corresponds to the transition of a photon of wavelength 275nm and also transitions of minimum and maximum wavelengths.
We know that the energy of a photon is given by,
$E=h\nu $ ……………………………………………. (1)
Where, h is the Planck’s constant given by,
$h=6.63\times {{10}^{-34}}{{m}^{2}}kg/s$
Where, $\nu $ is the frequency of the radiation emitted given by,
$\nu =\dfrac{c}{\lambda }$ ………………………………………………. (2)
Substituting (2) in (1), we get,
$E=\dfrac{hc}{\lambda }$ ……………………. (3)
Where c is the universal speed of light in vacuum given by,
$c=3\times {{10}^{8}}m/s$
Now, by substituting the values we get,
$E=\dfrac{6.63\times {{10}^{-34}}\times 3\times {{10}^{8}}}{275\times {{10}^{-9}}}$
$E=0.072\times {{10}^{-17}}J$ ……………………. (4)
We know that, one electron volt is given by,
$1eV=1.6\times {{10}^{-19}}J$
We could now convert (4) into electron volts.
$E=\dfrac{0.072\times {{10}^{-17}}}{1.6\times {{10}^{-19}}}eV$
$\therefore E=4.5eV$
Energy of emitted radiation is given by the difference of energy of the energy levels across which the transition takes place.
So the transition that corresponds to the transition of a photon of wavelength 275nm will be transition B whose energy of emitted radiation will be the same as that of the photon.
$0-\left( -4.5eV \right)=4.5eV$
From equation (3) we see that energy is inversely proportional to the wavelength of radiation. So the transition that emits radiation of least energy will have the maximum wavelength and the radiation with maximum energy will have minimum wavelength.
(a) Minimum energy emitted will be for transition A,
$0-\left( -2eV \right)=2eV$
So the maximum wavelength will also be for the radiation emitted in transition A.
(b) Maximum energy emitted will be for transition C,
$-2eV-\left( -10eV \right)=8eV$
So the minimum wavelength will also be for the radiation emitted in transition C.
Note:
In case you wonder what these energy levels are, they are the fixed distances from the nucleus of an atom where there is a possibility for the electrons to be found. Energy levels could be compared to steps of a staircase. The electrons can be present in one or the other energy level but not in between.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




