
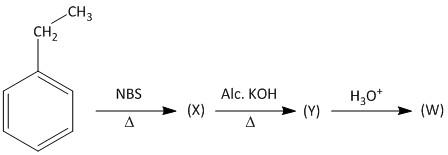
The end product ‘W’ in the following sequence of reaction is:
(A)
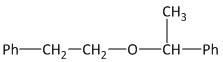
(B)
(C) $Ph - C{H_2} - O - C{H_2} - C{H_2} - Ph$
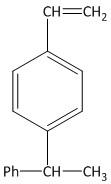
(D)

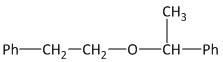


Answer
545.4k+ views
Hint: The \[NBS\] is a reagent used for benzylic bromination. The middle step is an elimination reaction. The final step is a hydration reaction of alkene.
Complete step by step answer:
The given starting material for the reaction sequence is ethyl benzene. It is an aromatic hydrocarbon which undergoes benzylic bromination upon heating with \[NBS\]. \[NBS\] is a brominating agent which contains bromine radicals. The bromine radical reacts with the hydrocarbon to produce more stabilized benzyl bromide. Thus the product of the reaction is (1-bromoethyl)benzene. The reaction is shown as
Step I:
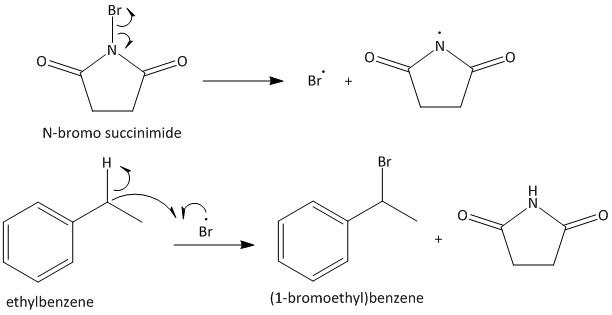
In the second step the alcoholic \[KOH\] is used to abstract the hydrogen from the methyl group of the benzyl bromide. The abstraction of the hydrogen from the compound results in dehydrobromination and produces styrene. The reaction is shown as
Step II:
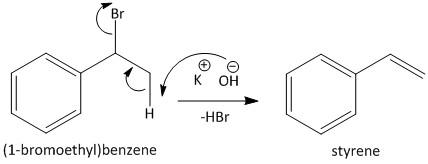
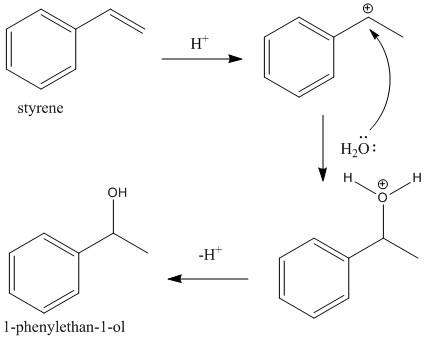
The third and the final step is the hydration of styrene molecules in presence of aqueous acid. At first the acid protonated the double bond of the styrene molecule to produce a more stable \[{2^o}\]carbocation (secondary). This then undergoes addition of water molecules to generate \[1\]-phenylethan-\[1\]-ol. The reaction is shown as
Step III:
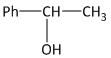
Hence the end product ‘W’ of the given sequence of reaction is \[1\]-phenylethan-\[1\]-ol, i.e. option B is the correct answer.
So, the correct answer is Option B.
Note: The reaction conditions and the abbreviations are to be kept in mind. The alc. is used for alcoholic \[KOH\] and aq. is used for aqueous \[KOH\]. Alc. \[KOH\] will result in elimination of the product but the aqueous \[KOH\] is used for hydroxylation i.e. addition of \[OH\].
Complete step by step answer:
The given starting material for the reaction sequence is ethyl benzene. It is an aromatic hydrocarbon which undergoes benzylic bromination upon heating with \[NBS\]. \[NBS\] is a brominating agent which contains bromine radicals. The bromine radical reacts with the hydrocarbon to produce more stabilized benzyl bromide. Thus the product of the reaction is (1-bromoethyl)benzene. The reaction is shown as
Step I:

In the second step the alcoholic \[KOH\] is used to abstract the hydrogen from the methyl group of the benzyl bromide. The abstraction of the hydrogen from the compound results in dehydrobromination and produces styrene. The reaction is shown as
Step II:

The third and the final step is the hydration of styrene molecules in presence of aqueous acid. At first the acid protonated the double bond of the styrene molecule to produce a more stable \[{2^o}\]carbocation (secondary). This then undergoes addition of water molecules to generate \[1\]-phenylethan-\[1\]-ol. The reaction is shown as
Step III:

Hence the end product ‘W’ of the given sequence of reaction is \[1\]-phenylethan-\[1\]-ol, i.e. option B is the correct answer.
So, the correct answer is Option B.
Note: The reaction conditions and the abbreviations are to be kept in mind. The alc. is used for alcoholic \[KOH\] and aq. is used for aqueous \[KOH\]. Alc. \[KOH\] will result in elimination of the product but the aqueous \[KOH\] is used for hydroxylation i.e. addition of \[OH\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




