
The dipole moment of $N{F_3}$ is less than $N{H_3}$ because of the following:
A.$N{H_3}$ forms associated molecules
B.$F$ is more electronegative than $N$
C.The resultant bond polarity is less
D.The resultant of individual polarities that is opposed by polarity of lone pairs in $N{F_3}$ .
Answer
576.6k+ views
Hint:Dipole moment is defined as the difference in electronegativity between two chemically bonded atoms.it can arise in any of the system when there is a separation of charge. It is denoted by the symbol $'d'$ .
Complete step by step answer:
-Dipole moment occurs when there is a difference in electronegativity between two chemically bonded atoms.
-It is commonly denoted by the symbol $\left( D \right)$ .
-It is also denoted as $'\mu '$
-The formula of dipole moment is as follows:
$\text{Dipole Moment} = charge \times \text{distance separation}$
$\mu = Q \times r$ .
-Dipole moment can be measured in Debye units $D$ .
The value of Debye unit is $D = 3.33564{{ }} \times {{ }}{10^{ - 30}}\;C.m.$
Where $C = Coulomb$, $m = meter$ .
-Properties of $NF_3$ and $NH_3$ are as follows:
-Structure of both the molecules is pyramidal.
-In $N{F_3}$ , fluorine has the tendency to accept electrons whereas in $N{H_3}$ , hydrogen is comparatively small and can easily donate its electron.
-Both the molecules have lone pairs of electrons and three bond pairs.
-Also they both are inorganic compounds.
Now, we will see the structure of $N{F_3}$ and $N{H_3}$ as follows:
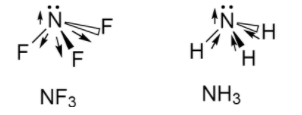
The dipole moment of $N{F_3}$ is $0.24D$ whereas the dipole moment of $N{H_3}$ is $1.46D$ .
In the case of $N{H_3}$ ,since the nitrogen is more electronegative than hydrogen, so it will try to pull the electrons from hydrogen atoms thus the polarity will be towards the nitrogen atom.
In the case of $N{F_3}$ ,since the fluorine being more electronegative than nitrogen will try to pull the electrons from nitrogen opposite from that of nitrogen, so the polarity is towards the fluorine.
So, the correct answer is option D) The resultant of individual polarities is opposed by the polarity of the lone pair in $N{F_3}$ .
Note:
In this dipole moment helps us to know the electronegativity of an element because if the dipole moment is greater than it means that electronegativity will be greater. Even by electronegativity we can guess whether the molecule has less or more dipole moment.
Complete step by step answer:
-Dipole moment occurs when there is a difference in electronegativity between two chemically bonded atoms.
-It is commonly denoted by the symbol $\left( D \right)$ .
-It is also denoted as $'\mu '$
-The formula of dipole moment is as follows:
$\text{Dipole Moment} = charge \times \text{distance separation}$
$\mu = Q \times r$ .
-Dipole moment can be measured in Debye units $D$ .
The value of Debye unit is $D = 3.33564{{ }} \times {{ }}{10^{ - 30}}\;C.m.$
Where $C = Coulomb$, $m = meter$ .
-Properties of $NF_3$ and $NH_3$ are as follows:
-Structure of both the molecules is pyramidal.
-In $N{F_3}$ , fluorine has the tendency to accept electrons whereas in $N{H_3}$ , hydrogen is comparatively small and can easily donate its electron.
-Both the molecules have lone pairs of electrons and three bond pairs.
-Also they both are inorganic compounds.
Now, we will see the structure of $N{F_3}$ and $N{H_3}$ as follows:
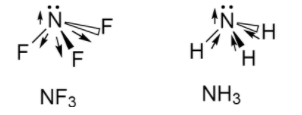
The dipole moment of $N{F_3}$ is $0.24D$ whereas the dipole moment of $N{H_3}$ is $1.46D$ .
In the case of $N{H_3}$ ,since the nitrogen is more electronegative than hydrogen, so it will try to pull the electrons from hydrogen atoms thus the polarity will be towards the nitrogen atom.
In the case of $N{F_3}$ ,since the fluorine being more electronegative than nitrogen will try to pull the electrons from nitrogen opposite from that of nitrogen, so the polarity is towards the fluorine.
So, the correct answer is option D) The resultant of individual polarities is opposed by the polarity of the lone pair in $N{F_3}$ .
Note:
In this dipole moment helps us to know the electronegativity of an element because if the dipole moment is greater than it means that electronegativity will be greater. Even by electronegativity we can guess whether the molecule has less or more dipole moment.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




