
The critical temperature of water is higher than that of ${{\text{O}}_2}$ because ${{\text{H}}_2}{\text{O}}$ molecule has:
${\text{A}}{\text{.}}$ Fewer electrons than ${{\text{O}}_2}$
${\text{B}}{\text{.}}$ Two covalent bonds
${\text{C}}{\text{.}}$ V-shape
${\text{D}}{\text{.}}$ Dipole moment
Answer
615.3k+ views
Hint- Here, we will proceed by defining the term critical temperature of any substance. Then, we will discuss the nature of the bond formed between the atoms of both the molecules i.e., water (${{\text{H}}_2}{\text{O}}$) and diatomic oxygen (${{\text{O}}_2}$).
Complete answer:
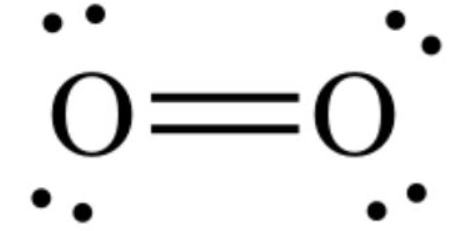
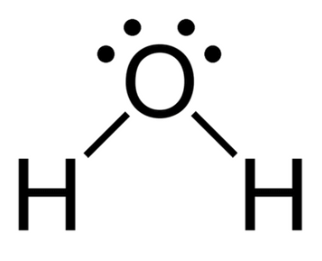
The critical temperature of a substance can be defined as the highest temperature at which the substance can exist as a liquid. The substance in its vapour or gaseous state can no longer be able to liquefy regardless of the amount of pressure applied to it at any temperatures above the critical temperature.
Since, we know that the force of attraction between the molecules will be lower if the substance is in gaseous or vapour state as compared to that substance in the liquid state.
If we observe water (${{\text{H}}_2}{\text{O}}$) molecule, we can say that this molecule consists of one oxygen atom and two hydrogen atoms. When hydrogen atom comes in the vicinity of oxygen atom, there occurs a tendency of the hydrogen atom to lose one electron to become electropositive and simultaneously, that electron is accepted by the oxygen atom to become electronegative in order to form a covalent bond between the hydrogen atom and the oxygen atom. Dipole moment is developed due to this polar nature of the water (${{\text{H}}_2}{\text{O}}$) molecule. The forces of attraction within the water molecules are high due to this high polar dipole moment and hence, its critical temperature is also high.
The oxygen-oxygen double bond is arranged symmetrically if we observe ${{\text{O}}_2}$ molecule and hence there is no overall dipole in the molecule. The diatomic oxygen molecule (${{\text{O}}_2}$) does not have polarity in the covalent bond because of equal electronegativity. This will lead to no polarity or dipole moment present in the molecule and its critical temperature is lower than that of water molecule.
Therefore, the critical temperature of water (${{\text{H}}_2}{\text{O}}$) is higher than that of diatomic oxygen molecule (${{\text{O}}_2}$) because ${{\text{H}}_2}{\text{O}}$ molecule has dipole moment.
Hence, option D is correct.
Note- Whenever there is a separation of charge, dipole moment occurs. It can occur between two ions in an ionic bond or between atoms in a covalent bond. The dipole moment arises due to the differences in electronegativity. The dipole moment is a measure of the polarity of the molecule.
Complete answer:
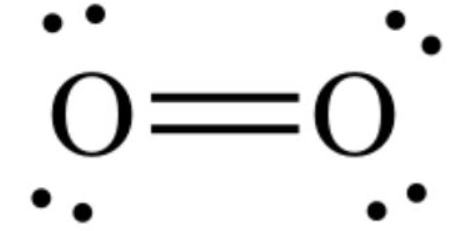

The critical temperature of a substance can be defined as the highest temperature at which the substance can exist as a liquid. The substance in its vapour or gaseous state can no longer be able to liquefy regardless of the amount of pressure applied to it at any temperatures above the critical temperature.
Since, we know that the force of attraction between the molecules will be lower if the substance is in gaseous or vapour state as compared to that substance in the liquid state.
If we observe water (${{\text{H}}_2}{\text{O}}$) molecule, we can say that this molecule consists of one oxygen atom and two hydrogen atoms. When hydrogen atom comes in the vicinity of oxygen atom, there occurs a tendency of the hydrogen atom to lose one electron to become electropositive and simultaneously, that electron is accepted by the oxygen atom to become electronegative in order to form a covalent bond between the hydrogen atom and the oxygen atom. Dipole moment is developed due to this polar nature of the water (${{\text{H}}_2}{\text{O}}$) molecule. The forces of attraction within the water molecules are high due to this high polar dipole moment and hence, its critical temperature is also high.
The oxygen-oxygen double bond is arranged symmetrically if we observe ${{\text{O}}_2}$ molecule and hence there is no overall dipole in the molecule. The diatomic oxygen molecule (${{\text{O}}_2}$) does not have polarity in the covalent bond because of equal electronegativity. This will lead to no polarity or dipole moment present in the molecule and its critical temperature is lower than that of water molecule.
Therefore, the critical temperature of water (${{\text{H}}_2}{\text{O}}$) is higher than that of diatomic oxygen molecule (${{\text{O}}_2}$) because ${{\text{H}}_2}{\text{O}}$ molecule has dipole moment.
Hence, option D is correct.
Note- Whenever there is a separation of charge, dipole moment occurs. It can occur between two ions in an ionic bond or between atoms in a covalent bond. The dipole moment arises due to the differences in electronegativity. The dipole moment is a measure of the polarity of the molecule.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




