
The critical micelle concentration (CMC) is defined as:
A. the concentration at which micellization starts
B. the concentration at which micelle starts behaving like an electrolyte
C. the concentration at which dispersed phase is separated from dispersion medium
D. the concentration at which a colloid is converted to suspension
Answer
590.4k+ views
Hint: Some substances like associated colloids which at low concentrations behave as normal electrolytes but at higher concentrations, they tend to exhibit the colloidal behaviour due to the formation of aggregates. These aggregated particles are called micelles. There are mechanisms and properties of micelles which clear out everything.
Complete step by step answer:
Let us first discuss the mechanism of micelle formation is
Like, Soap is sodium or potassium salt of a higher fatty acid and which is represented as $\text{RCO}{{\text{O}}^{-}}\text{N}{{\text{a}}^{+}}$. When it is dissolved in water, it gets dissociated into $\text{N}{{\text{a}}^{+}}$ and $\text{RCO}{{\text{O}}^{-}}$ ions. There are two parts in $\text{RCO}{{\text{O}}^{-}}$ ion one is a long hydrocarbon chain $\text{R}$ or the nonpolar tail which is hydrophobic and a polar group attached $\text{CO}{{\text{O}}^{-}}$ or the polar-ionic head which is hydrophilic. The structure seems like this-
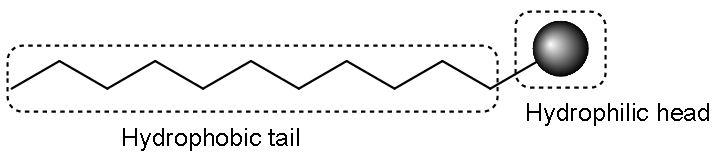
Therefore, the $\text{RCO}{{\text{O}}^{-}}$ ions are present on the surface of water with the hydrocarbon chain $\text{R}$ staying away from the water and remain at the surface and the $\text{CO}{{\text{O}}^{-}}$ group in water. At critical micelle concentration, the anions are dragged into the solution and aggregated to form a spherical shape with their hydrocarbon chain pointing towards the centre of the sphere with $\text{CO}{{\text{O}}^{-}}$ part remaining outward on the surface of the sphere. The aggregate which is formed is called ‘ionic micelle’. These micelles may contain as many as 100 particles.
Kraft temperature is the temperature above which the formation of micelles takes place and above a particular concentration which is called critical micelle concentration (CMC).
The correct answer is option ‘a’. The critical micelle concentration (CMC) is defined as the concentration at which micellization starts.
So, the correct answer is “Option A”.
Note: The temperature effect varies the critical micelle concentration value with the type of surfactant molecules. The shape and size of the micelles can be changed by changing the chemical structure of the surfactant as well as by changing the conditions of solution such as temperature and electrolytes addition.
Complete step by step answer:
Let us first discuss the mechanism of micelle formation is
Like, Soap is sodium or potassium salt of a higher fatty acid and which is represented as $\text{RCO}{{\text{O}}^{-}}\text{N}{{\text{a}}^{+}}$. When it is dissolved in water, it gets dissociated into $\text{N}{{\text{a}}^{+}}$ and $\text{RCO}{{\text{O}}^{-}}$ ions. There are two parts in $\text{RCO}{{\text{O}}^{-}}$ ion one is a long hydrocarbon chain $\text{R}$ or the nonpolar tail which is hydrophobic and a polar group attached $\text{CO}{{\text{O}}^{-}}$ or the polar-ionic head which is hydrophilic. The structure seems like this-

Therefore, the $\text{RCO}{{\text{O}}^{-}}$ ions are present on the surface of water with the hydrocarbon chain $\text{R}$ staying away from the water and remain at the surface and the $\text{CO}{{\text{O}}^{-}}$ group in water. At critical micelle concentration, the anions are dragged into the solution and aggregated to form a spherical shape with their hydrocarbon chain pointing towards the centre of the sphere with $\text{CO}{{\text{O}}^{-}}$ part remaining outward on the surface of the sphere. The aggregate which is formed is called ‘ionic micelle’. These micelles may contain as many as 100 particles.
Kraft temperature is the temperature above which the formation of micelles takes place and above a particular concentration which is called critical micelle concentration (CMC).
The correct answer is option ‘a’. The critical micelle concentration (CMC) is defined as the concentration at which micellization starts.
So, the correct answer is “Option A”.
Note: The temperature effect varies the critical micelle concentration value with the type of surfactant molecules. The shape and size of the micelles can be changed by changing the chemical structure of the surfactant as well as by changing the conditions of solution such as temperature and electrolytes addition.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




