
The correct structure of the drug paracetamol is:
A.

B.
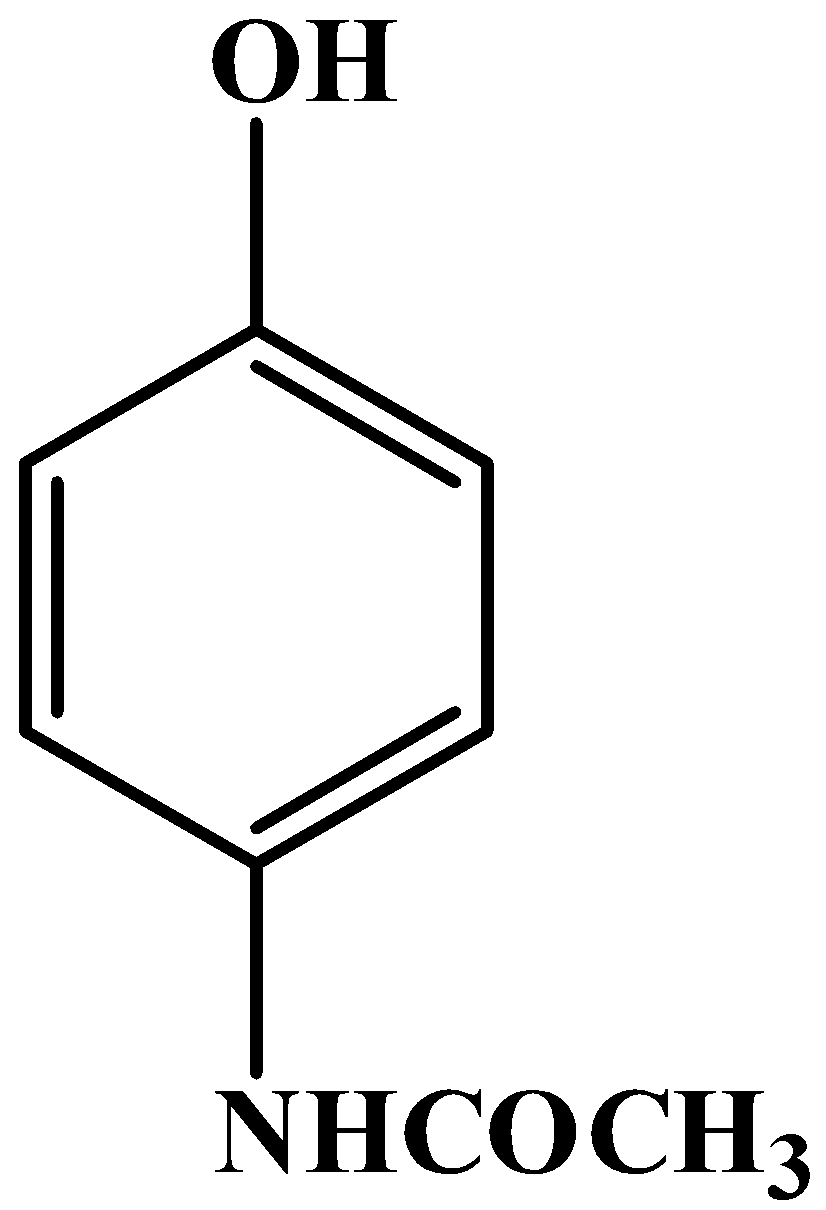
C.
D.





Answer
586.2k+ views
Hint: The structure of paracetamol is a like a substituted phenol. Apart from the hydroxyl group it also contains an acyl group linked to nitrogen atom.
Complete step by step answer:
To know the structure of any compound, we always start with its scientific name. As it turns out, the IUPAC name of this drug is $\text{N-}\left( \text{4-hydroxyphenyl} \right)\text{acetamide}$. It is also commonly called acetaminophen.
Let’s derive the structure of paracetamol from its name in a stepwise fashion.
- The primary carbon chain here is an amine. And we know its name; it is an acetamide. It structure is as follows:

- There is a phenyl group attached to the above carbon chain at the nitrogen atom. This phenyl group has a hydroxyl group attached to it at the fourth carbon. So technically, it’s a phenol. Now, we all know the structure of phenol but we will still see it. It is as follows:
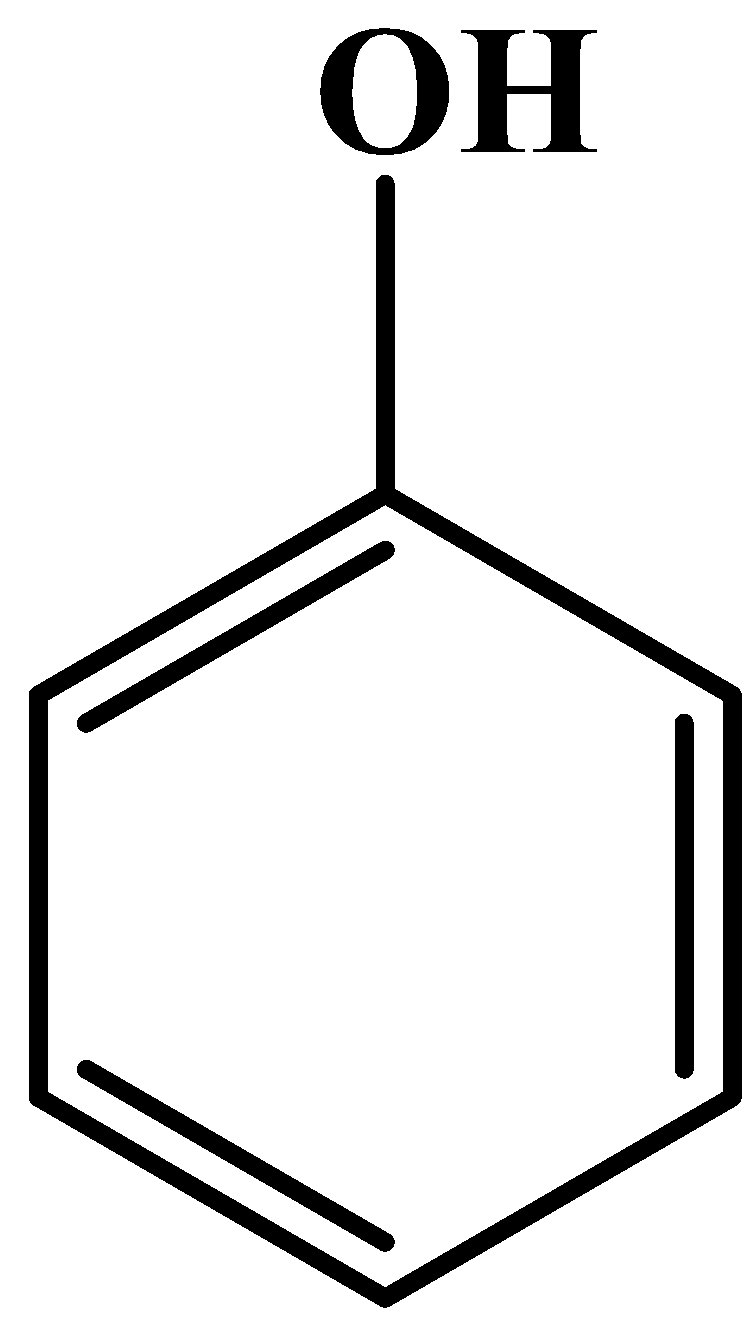
- So, putting them at their respective places we get the following structure of paracetamol:

So, the correct answer is “Option B”.
Additional Information:
- Paracetamol is a drug which is commonly prescribed for mild to moderate pain and fever. The type of pain can include, headache, body ache or aching of any particular muscle.
- This drug is also effective against menstrual pain which occurs in females.
Note: Solving this type of question, requires the exact knowledge of the chemical compound’s IUPAC name, so that one can at least derive the structure as we did above.
Any knowledge of one or more of its functional groups can also help in this process.
Complete step by step answer:
To know the structure of any compound, we always start with its scientific name. As it turns out, the IUPAC name of this drug is $\text{N-}\left( \text{4-hydroxyphenyl} \right)\text{acetamide}$. It is also commonly called acetaminophen.
Let’s derive the structure of paracetamol from its name in a stepwise fashion.
- The primary carbon chain here is an amine. And we know its name; it is an acetamide. It structure is as follows:

- There is a phenyl group attached to the above carbon chain at the nitrogen atom. This phenyl group has a hydroxyl group attached to it at the fourth carbon. So technically, it’s a phenol. Now, we all know the structure of phenol but we will still see it. It is as follows:

- So, putting them at their respective places we get the following structure of paracetamol:

So, the correct answer is “Option B”.
Additional Information:
- Paracetamol is a drug which is commonly prescribed for mild to moderate pain and fever. The type of pain can include, headache, body ache or aching of any particular muscle.
- This drug is also effective against menstrual pain which occurs in females.
Note: Solving this type of question, requires the exact knowledge of the chemical compound’s IUPAC name, so that one can at least derive the structure as we did above.
Any knowledge of one or more of its functional groups can also help in this process.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




