
The correct structure of monomers of buna-N is :
(A)
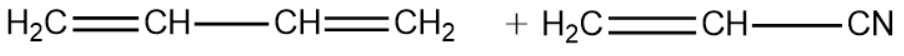
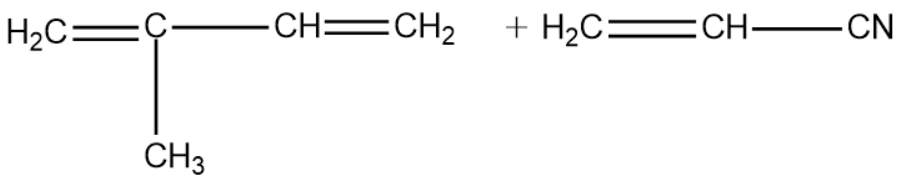
(B)
(C)
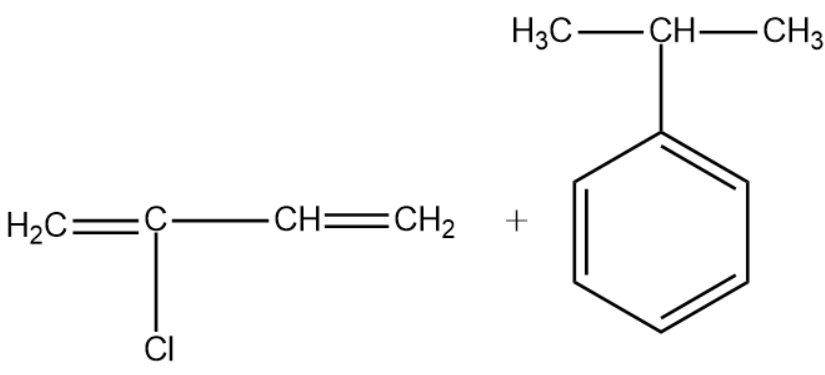
(D)
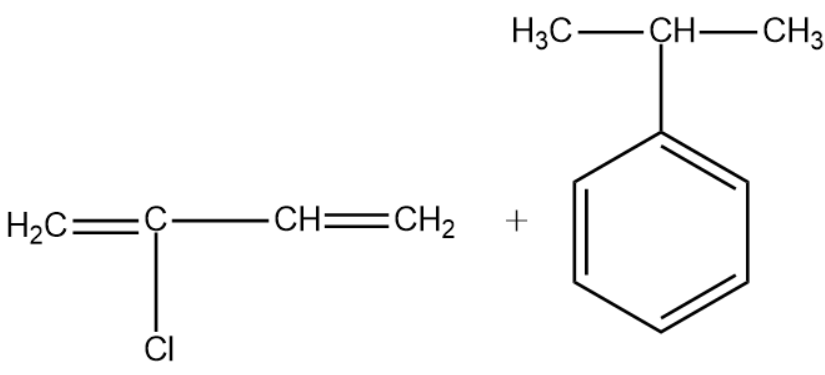
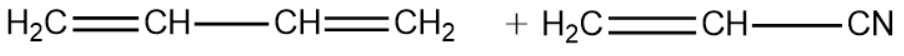
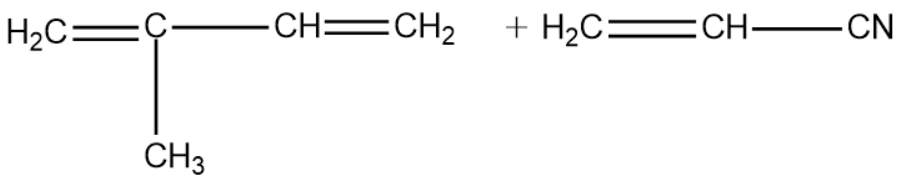


Answer
584.4k+ views
Hint: Buna-N is a polymer made up of 2 polymers. This means that it is a copolymer. The reaction mechanism involves formation of free radicals. So, check for compounds which can easily generate free radicals. Identify the names of the monomers and then draw the structures accordingly.
Complete step-by-step answer:
Polymerization is a process of reacting monomer molecules together in a chemical reaction to form long polymer chains .
In chemical compounds, polymerization can occur by various types of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and the steric effects that are present.
Copolymerisation is the type of polymerisation reaction involving more than one type of monomer. The number of monomers involved in the reaction is usually 2.
Buna-N is one such polymer that is formed by copolymerisation. The monomers of the polymer are 1,3-Butadiene and vinyl cyanide.
We will now draw the structures of each monomer and then identify the correct answer.
1,3-Butadiene:
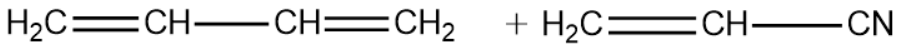
Vinyl Cyanide:
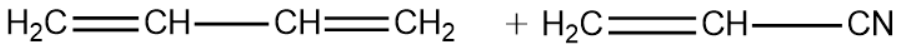
We will now write the polymerisation reaction of the above two monomeric units and see the formation of the Buna-N polymer.
From the above explanation we can conclude that the correct answer is option (A).
Note: In simple polymerizations, alkenes form polymers through relatively simple free radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize and the steric hindrance offered by neighboring groups.
Complete step-by-step answer:
Polymerization is a process of reacting monomer molecules together in a chemical reaction to form long polymer chains .
In chemical compounds, polymerization can occur by various types of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and the steric effects that are present.
Copolymerisation is the type of polymerisation reaction involving more than one type of monomer. The number of monomers involved in the reaction is usually 2.
Buna-N is one such polymer that is formed by copolymerisation. The monomers of the polymer are 1,3-Butadiene and vinyl cyanide.
We will now draw the structures of each monomer and then identify the correct answer.
1,3-Butadiene:
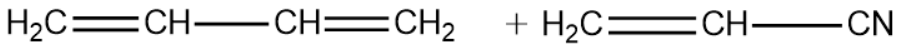
Vinyl Cyanide:
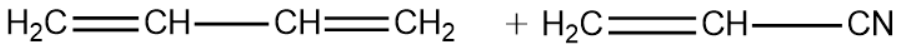
We will now write the polymerisation reaction of the above two monomeric units and see the formation of the Buna-N polymer.
From the above explanation we can conclude that the correct answer is option (A).
Note: In simple polymerizations, alkenes form polymers through relatively simple free radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize and the steric hindrance offered by neighboring groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




