
The correct statement(s) for orthoboric acid is/are :
a.) It behaves as a weak acid in water due to self-ionization
b.) Acidity of its aqueous solution increases upon addition of ethylene glycol
c.) It has a three-dimensional structure due to hydrogen bonding
d.) It is a weak electrolyte in water
Answer
590.4k+ views
Hint: The orthoboric acid is a weak monobasic compound. After accepting $O{H^ - }$ ions from water even then it can not complete its octet. It is a weak lewis acid. It has square planar geometry even after hydrogen bonding.
Complete step by step answer:
First, we will see what is orthoboric acid.
It is also called hydrogen borate or boric acid is a lewis acid of boron. It is weak and monobasic in nature.
Let us see the options given to us and analyse them one by one.
The first option says that it behaves as a weak acid in water due to self-ionization.
Now let us see the reaction of boric acid with water.
${H_3}B{O_3} + 2{H_2}O \to {[B{(OH)_4}]^ - } + {H_3}{O^ + }$
From the reaction it is clear that it is accepting $O{H^ - }$ from water molecules and thus it does not self ionize. So, the option a.) is not correct.
Now, the option b.) says that acidity of its aqueous solution increases upon addition of ethylene glycol
For this let us see the reaction of orthoboric acid with ethylene glycol which is as-
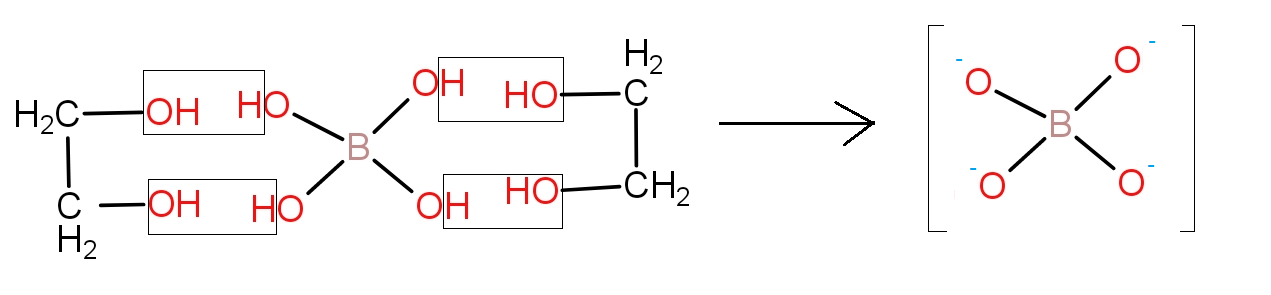
The product formed is stable. So, the reaction will occur and thus, the acidity of its aqueous solution will increase upon addition of ethylene glycol.
So, the option b.) is the correct answer.
The option c.) is that it has a three-dimensional structure due to hydrogen bonding.
This statement is wrong because it has planar structure not three-dimensional.
The last option is that it is a weak electrolyte in water.
This statement is true because it is a weak acid. Thus, it will be weak electrolyte.
So, the option d.) is also correct. So, the correct answer is “Option B and D”.
Note: Boric acid is commonly used as antiseptic, insecticide and neutron absorber. As the acidity of boric acid increases in presence of polyols. With the different concentrations of mannitol, the boric acid shows pKa value of five orders of magnitude. This property is used to determine boron content in aqueous solution in analytical chemistry.
Complete step by step answer:
First, we will see what is orthoboric acid.
It is also called hydrogen borate or boric acid is a lewis acid of boron. It is weak and monobasic in nature.
Let us see the options given to us and analyse them one by one.
The first option says that it behaves as a weak acid in water due to self-ionization.
Now let us see the reaction of boric acid with water.
${H_3}B{O_3} + 2{H_2}O \to {[B{(OH)_4}]^ - } + {H_3}{O^ + }$
From the reaction it is clear that it is accepting $O{H^ - }$ from water molecules and thus it does not self ionize. So, the option a.) is not correct.
Now, the option b.) says that acidity of its aqueous solution increases upon addition of ethylene glycol
For this let us see the reaction of orthoboric acid with ethylene glycol which is as-

The product formed is stable. So, the reaction will occur and thus, the acidity of its aqueous solution will increase upon addition of ethylene glycol.
So, the option b.) is the correct answer.
The option c.) is that it has a three-dimensional structure due to hydrogen bonding.
This statement is wrong because it has planar structure not three-dimensional.
The last option is that it is a weak electrolyte in water.
This statement is true because it is a weak acid. Thus, it will be weak electrolyte.
So, the option d.) is also correct. So, the correct answer is “Option B and D”.
Note: Boric acid is commonly used as antiseptic, insecticide and neutron absorber. As the acidity of boric acid increases in presence of polyols. With the different concentrations of mannitol, the boric acid shows pKa value of five orders of magnitude. This property is used to determine boron content in aqueous solution in analytical chemistry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




