
The correct statements about the following sugars X and Y are:
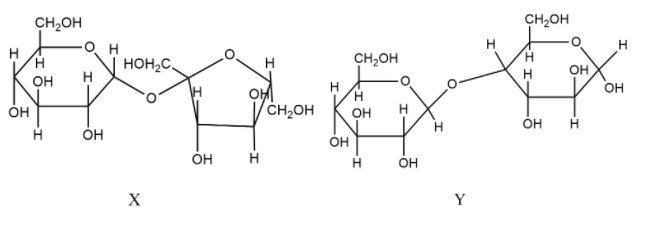
(A) X is a reducing sugar, Y is a non-reducing sugar.
(B) X is a non-reducing sugar, Y is a reducing sugar.
(C) The glycosidic linkages in X and Y are $\alpha $ and $\beta $ respectively.
(D) The glycosidic linkages in X and Y are $\beta $ and $\alpha $ respectively.

Answer
581.4k+ views
Hint: Think about the topic of carbohydrates. Two disaccharides are given in the question and four options are given. We need to determine whether they are reducing sugars or not. Also, we need to find out the type of glycosidic linkages present in both the sugars and then choose the correct option.
Complete answer:
- Let’s start by taking a look at the given compounds, X and Y.
- A carbohydrate is said to be a reducing sugar if the carbon at first position contains a free hydroxyl group (-OH) attached to it. If at C-1 position, a free hydroxyl group is absent then the sugar is a non-reducing sugar.
- To find out the type of linkage present in the disaccharide, we need to check the monosaccharide of which C-1 position is forming the linkage. If the monosaccharide is $\alpha $ then the linkage is said to be $\alpha $-linkage and if the monosaccharide is $\beta $ then the linkage is said to be $\beta $-linkage.
- Now, let’s see the compounds X and Y one by one.
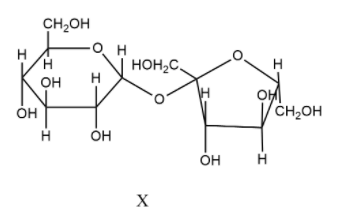
- In X, there are no free hydroxyl groups present and so, this is a non-reducing sugar. The monosaccharide forming the linkage is glucose which is present as $\alpha $-glucose. Therefore, an $\alpha $-linkage is formed.
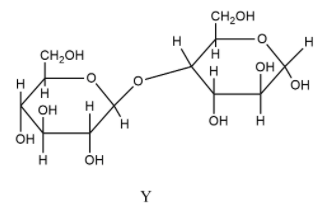
- In Y, there is one free hydroxyl group present , so this is a reducing sugar. The monosaccharide forming the linkage is glucose which is present as $\beta $-glucose. Therefore, an $\beta $-linkage is formed.
- Therefore, X is a non-reducing sugar, Y is a reducing sugar and the glycosidic linkages in X and Y are $\alpha $ and $\beta $ respectively.
Therefore, the correct statements are option (B) and (C).
Note:
Remember for a sugar to be classified as a reducing sugar, at least one free hydroxyl group must be present at C-1 position. If no free hydroxyl groups are present then the sugar is a non-reducing sugar. If the C-1 atom of the monosaccharide forming the linkage is an $\alpha $-anomer then the linkage will be $\alpha $-linkage and if the C-1 atom of the monosaccharide forming the linkage is a $\beta $-anomer then the linkage will be $\beta $-linkage.
Complete answer:
- Let’s start by taking a look at the given compounds, X and Y.
- A carbohydrate is said to be a reducing sugar if the carbon at first position contains a free hydroxyl group (-OH) attached to it. If at C-1 position, a free hydroxyl group is absent then the sugar is a non-reducing sugar.
- To find out the type of linkage present in the disaccharide, we need to check the monosaccharide of which C-1 position is forming the linkage. If the monosaccharide is $\alpha $ then the linkage is said to be $\alpha $-linkage and if the monosaccharide is $\beta $ then the linkage is said to be $\beta $-linkage.
- Now, let’s see the compounds X and Y one by one.

- In X, there are no free hydroxyl groups present and so, this is a non-reducing sugar. The monosaccharide forming the linkage is glucose which is present as $\alpha $-glucose. Therefore, an $\alpha $-linkage is formed.

- In Y, there is one free hydroxyl group present , so this is a reducing sugar. The monosaccharide forming the linkage is glucose which is present as $\beta $-glucose. Therefore, an $\beta $-linkage is formed.
- Therefore, X is a non-reducing sugar, Y is a reducing sugar and the glycosidic linkages in X and Y are $\alpha $ and $\beta $ respectively.
Therefore, the correct statements are option (B) and (C).
Note:
Remember for a sugar to be classified as a reducing sugar, at least one free hydroxyl group must be present at C-1 position. If no free hydroxyl groups are present then the sugar is a non-reducing sugar. If the C-1 atom of the monosaccharide forming the linkage is an $\alpha $-anomer then the linkage will be $\alpha $-linkage and if the C-1 atom of the monosaccharide forming the linkage is a $\beta $-anomer then the linkage will be $\beta $-linkage.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




