
The correct formula of permanganic acid is:
A. $HMn{{O}_{4}}$
B. $HMn{{O}_{5}}$
C. ${{H}_{2}}Mn{{O}_{4}}$
D. ${{H}_{2}}Mn{{O}_{3}}$
Answer
570k+ views
Hint: Permanganic acid is an inorganic oxyacid derived from dehydrates. It is also Conjugate acid of Permanganates salts.
Complete Solution :
Permanganic acid is manufactured by the reaction of dilute sulphuric acid with Barium permanganate leaving permanganic acid and Barium Sulphate.
$Ba{{\left( Mn{{O}_{4}} \right)}_{2}}+{{H}_{2}}S{{O}_{4}}\to 2HMn{{O}_{4}}+BaS{{O}_{4}}$
From here, we can see that the formula of Permanganic acid obtained is $HMn{{O}_{4}}$. It can also be prepared with the help of hydrofluorosilicic acid with potassium permanganate through electrolysis. It is prepared at lower temperatures as a dihydrate and then they are further obtained from it.
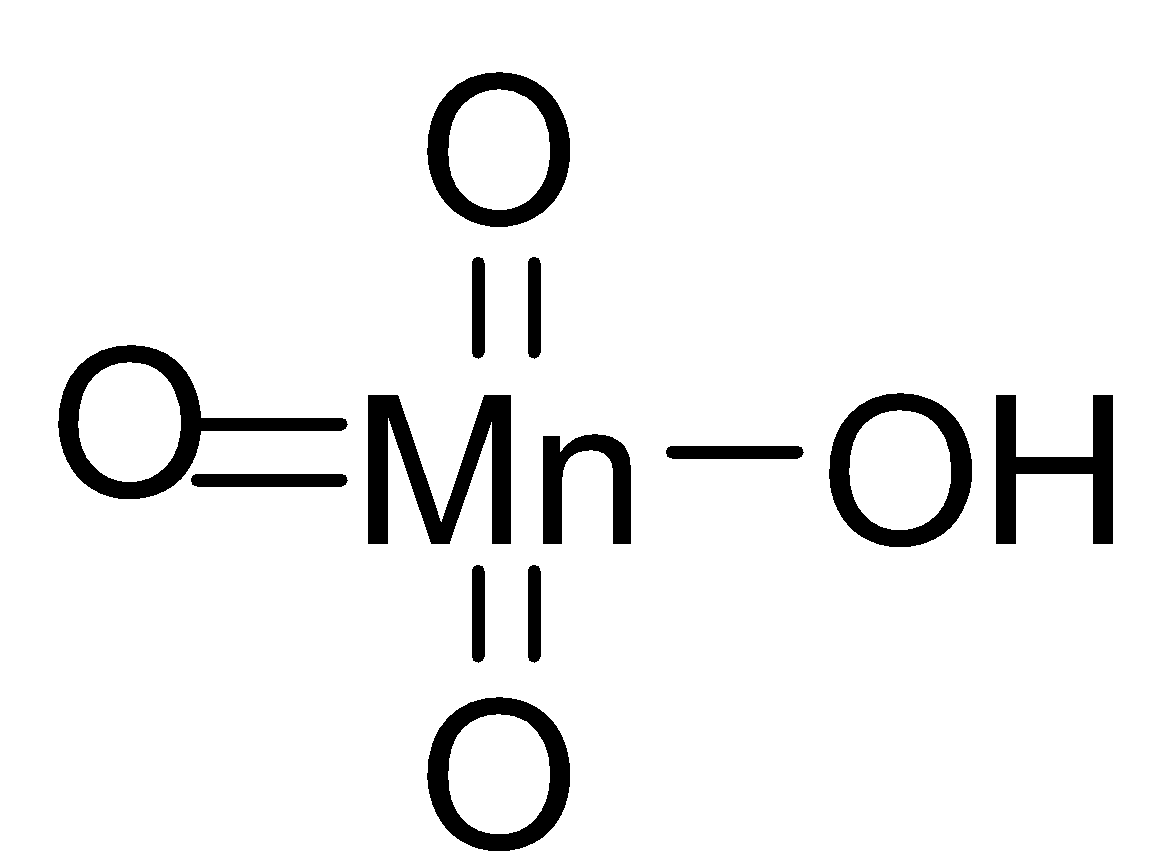
- The structure of permanganic acid is in tetrahedral form but it is not verified yet like peroxide. If the $HMn{{O}_{4}}$ is deprotonated then it forms purple coloured permanganates, mostly Potassium Permanganate. The solution of Permanganic acid is very unstable and they degenerate into manganese dioxide, oxygen and water. The decomposition is mostly accelerated and catalysed by the initially formed manganese oxide and decomposition is also affected by heat, light and acids.
So, the correct answer is “Option A”.
Note: The sulphuric acid used in the preparation of permanganic acid should be very dilute rather concentrated as the concentrated sulphuric acid will evolve Ozone. Although, this can be used to prepare Ozone chemically with the reaction of potassium permanganate with the concentration between 50-80% of sulphuric acid. However, if someone uses concentration above 80% then it can lead to the formation of a dangerous intermediate which is explosive in nature called manganese heptoxide.
Complete Solution :
Permanganic acid is manufactured by the reaction of dilute sulphuric acid with Barium permanganate leaving permanganic acid and Barium Sulphate.
$Ba{{\left( Mn{{O}_{4}} \right)}_{2}}+{{H}_{2}}S{{O}_{4}}\to 2HMn{{O}_{4}}+BaS{{O}_{4}}$
From here, we can see that the formula of Permanganic acid obtained is $HMn{{O}_{4}}$. It can also be prepared with the help of hydrofluorosilicic acid with potassium permanganate through electrolysis. It is prepared at lower temperatures as a dihydrate and then they are further obtained from it.

- The structure of permanganic acid is in tetrahedral form but it is not verified yet like peroxide. If the $HMn{{O}_{4}}$ is deprotonated then it forms purple coloured permanganates, mostly Potassium Permanganate. The solution of Permanganic acid is very unstable and they degenerate into manganese dioxide, oxygen and water. The decomposition is mostly accelerated and catalysed by the initially formed manganese oxide and decomposition is also affected by heat, light and acids.
So, the correct answer is “Option A”.
Note: The sulphuric acid used in the preparation of permanganic acid should be very dilute rather concentrated as the concentrated sulphuric acid will evolve Ozone. Although, this can be used to prepare Ozone chemically with the reaction of potassium permanganate with the concentration between 50-80% of sulphuric acid. However, if someone uses concentration above 80% then it can lead to the formation of a dangerous intermediate which is explosive in nature called manganese heptoxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




