
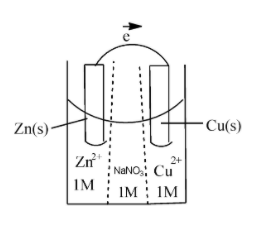
The concentration of $Z{{n}^{2+}}$in first compartment after passage of 0.1F charge will be:

Answer
569.7k+ views
Hint: The relationship between the quantity of electricity passed and the quantity of a substance liberated at the electrode is given in the form of Faraday's laws of electrolysis.
Complete step by step answer:
1. Faraday's First Law of Electrolysis. This law states that the mass of a substance liberated at the electrode is directly proportional to the quantity of electricity passed through the electrolyte.
\[mass=Z\times I\times T\,or\,\dfrac{EIT}{nF}\]
Thus equivalent of a substance is the amount of substance liberated at the electrode when current of one ampere is passed through the electrolyte for one second.
2. Faraday's Second Law of Electrolysis.
This law states that the amounts of different substances liberated by the same quantity of electricity passing through their electrolytic solution are directly proportional to their chemical equivalent masses (chemical equivalent mass of metal can be obtained by dividing its atomic mass with the number of electrons required to reduce its cation). The law can also be stated as follows; when same quantity of electricity is passed through different electrolytes connected in series then the masses of the substances liberated at the electrodes are in the ratio of their chemical equivalent masses (atomic mass + Number of electrons required to form the product) or the ratio of their electrochemical equivalents.
For example, if the two electrolytic cells A and B are connected in series and same quantity of electricity is passed through the cells. Then the ratio of the mass of copper deposited at cathode in electrolytic cell B to that of silver deposited in cell A is equal to the ratio of their chemical equivalent masses.
\[\dfrac{{{W}_{1}}}{{{E}_{1}}}=\dfrac{{{W}_{2}}}{{{E}_{2}}}=\dfrac{{{W}_{3}}}{{{E}_{3}}}=.....\dfrac{{{W}_{n}}}{{{E}_{n}}}\]
Now, each copper ($C{{u}^{2+}}$) ion requires 2 electrons to form Cu and each $A{{g}^{+}}$ needs 1 electron to form Ag. Thus, the chemical equivalent mass of Cu is $\dfrac{63.5}{2}$ and that of $Ag$ is $\dfrac{108}{1}$.
As we pass electricity, Zinc gets oxidised and concentration of zinc in solution increases.
By passing 0.1F of electricity it's concentration increases by $\dfrac{0.1}{4}$ $= 0.025M$.
So now conc. of $ZnS{{O}_{4}}$ is $1.025M$. So it is the answer.
Note: Faraday, also known as the faraday constant, is the unit of electricity, which is used in the study of electrochemical reactions and is equal to the amount of electric charge which liberates one gram equivalent of any ion from an electrolytic solution.
Complete step by step answer:
1. Faraday's First Law of Electrolysis. This law states that the mass of a substance liberated at the electrode is directly proportional to the quantity of electricity passed through the electrolyte.
\[mass=Z\times I\times T\,or\,\dfrac{EIT}{nF}\]
Thus equivalent of a substance is the amount of substance liberated at the electrode when current of one ampere is passed through the electrolyte for one second.
2. Faraday's Second Law of Electrolysis.
This law states that the amounts of different substances liberated by the same quantity of electricity passing through their electrolytic solution are directly proportional to their chemical equivalent masses (chemical equivalent mass of metal can be obtained by dividing its atomic mass with the number of electrons required to reduce its cation). The law can also be stated as follows; when same quantity of electricity is passed through different electrolytes connected in series then the masses of the substances liberated at the electrodes are in the ratio of their chemical equivalent masses (atomic mass + Number of electrons required to form the product) or the ratio of their electrochemical equivalents.
For example, if the two electrolytic cells A and B are connected in series and same quantity of electricity is passed through the cells. Then the ratio of the mass of copper deposited at cathode in electrolytic cell B to that of silver deposited in cell A is equal to the ratio of their chemical equivalent masses.
\[\dfrac{{{W}_{1}}}{{{E}_{1}}}=\dfrac{{{W}_{2}}}{{{E}_{2}}}=\dfrac{{{W}_{3}}}{{{E}_{3}}}=.....\dfrac{{{W}_{n}}}{{{E}_{n}}}\]
Now, each copper ($C{{u}^{2+}}$) ion requires 2 electrons to form Cu and each $A{{g}^{+}}$ needs 1 electron to form Ag. Thus, the chemical equivalent mass of Cu is $\dfrac{63.5}{2}$ and that of $Ag$ is $\dfrac{108}{1}$.
As we pass electricity, Zinc gets oxidised and concentration of zinc in solution increases.
By passing 0.1F of electricity it's concentration increases by $\dfrac{0.1}{4}$ $= 0.025M$.
So now conc. of $ZnS{{O}_{4}}$ is $1.025M$. So it is the answer.
Note: Faraday, also known as the faraday constant, is the unit of electricity, which is used in the study of electrochemical reactions and is equal to the amount of electric charge which liberates one gram equivalent of any ion from an electrolytic solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




