
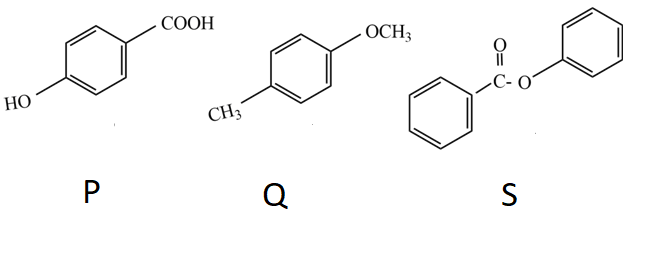
The compound P, Q, and S were separately subjected to nitration using $HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}$ mixture. The major product formed in each case respectively is:


Answer
573.9k+ views
Hint: In a chemical reaction, the functional group attached to a compound is replaced by an electrophile, and then the reactions are known as electrophilic substitution reactions. The functional group which is replaced by a hydrogen atom is substituted in a chemical compound. Organic compounds involve two types of primary electrophilic substitution reactions which are electrophilic aromatic substitution and aliphatic substitution reactions.
Complete Solution :
Benzene ring involved in substitution reaction is two types based on the type of substituent that ring carries, which are activated rings and deactivated rings.
If the substituent on the ring which groups are donating electrons is an activated ring and group withdraws electrons is a deactivated ring.
Example of activating groups of decreasing order:
$-N{{H}_{2}} > -N{{R}_{2}} > -OH > -OR > -NHCOR > -C{{H}_{3}}$
Example of deactivating groups with relative order from highest to lowest deactivating groups:
$-N{{O}_{2}} > -C{{F}_{3}} > -COR > -CN > -COOR > -S{{O}_{3}}H$
The activating group in the benzene ring directs the reaction to the Ortho or Para position and the deactivating group in the benzene ring directs the reaction to the Meta position.
(i) When compound P reacted with $HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}$ the mixture, the major product formed is,
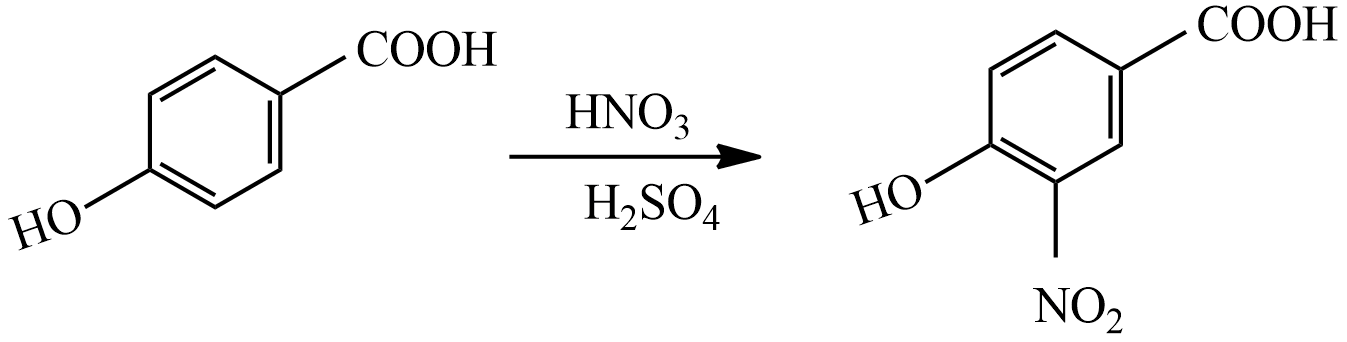
-OH group is an activating group and –COOH deactivating group, hence electrophile attack to the ortho position of the activating group –OH.
(ii) When compound Q reacted with $HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}$ the mixture, the major product formed is,
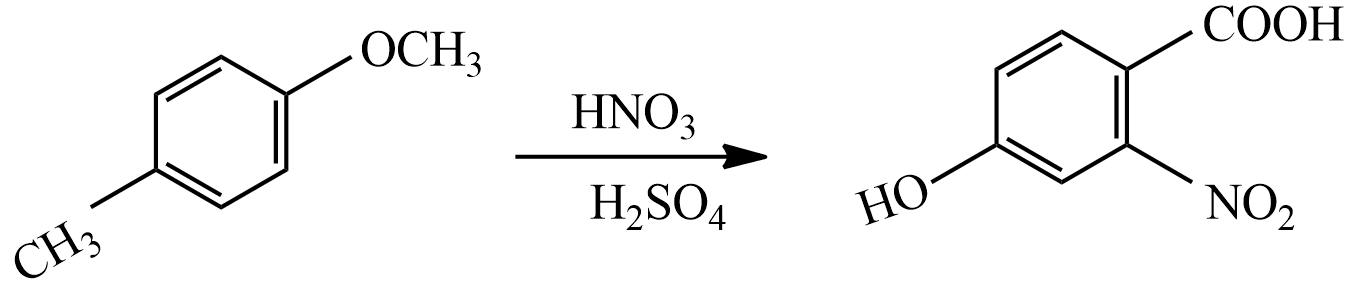
Both $-OC{{H}_{3}}$ and $-C{{H}_{3}}$ groups are activating groups which are directing Ortho or Para positions. But $-OC{{H}_{3}}$ group is a strong activating group than $-C{{H}_{3}}$ , so electrophile $N{{O}_{2}}^{-}$ attacked to Ortho position.
(iii) When compound S reacted with $HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}$ the mixture, the major product formed is,
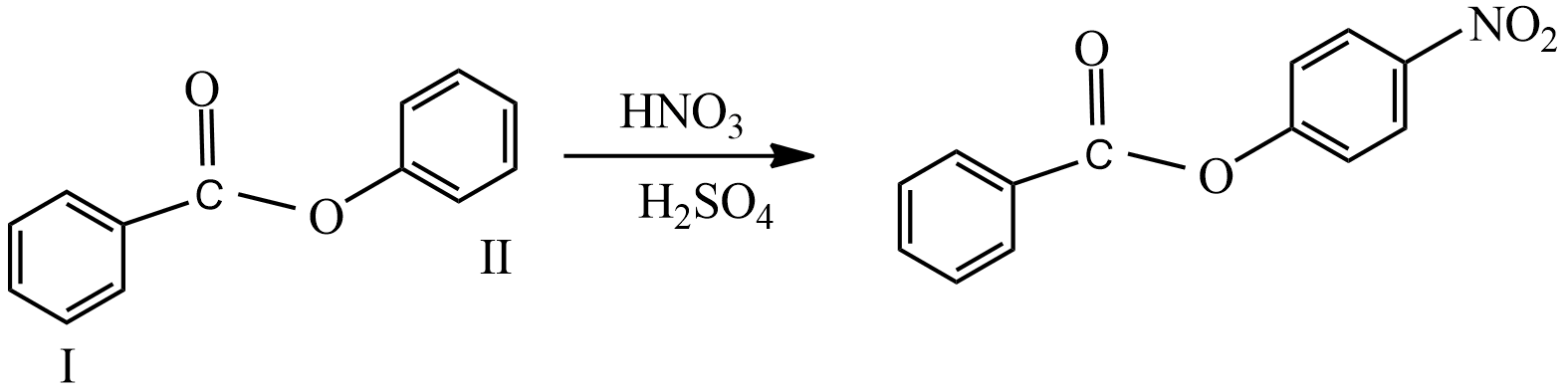
The ring I, is deactivated and ring II is activated, hence electrophile attack at Para position of ring II which is a less hindered position.
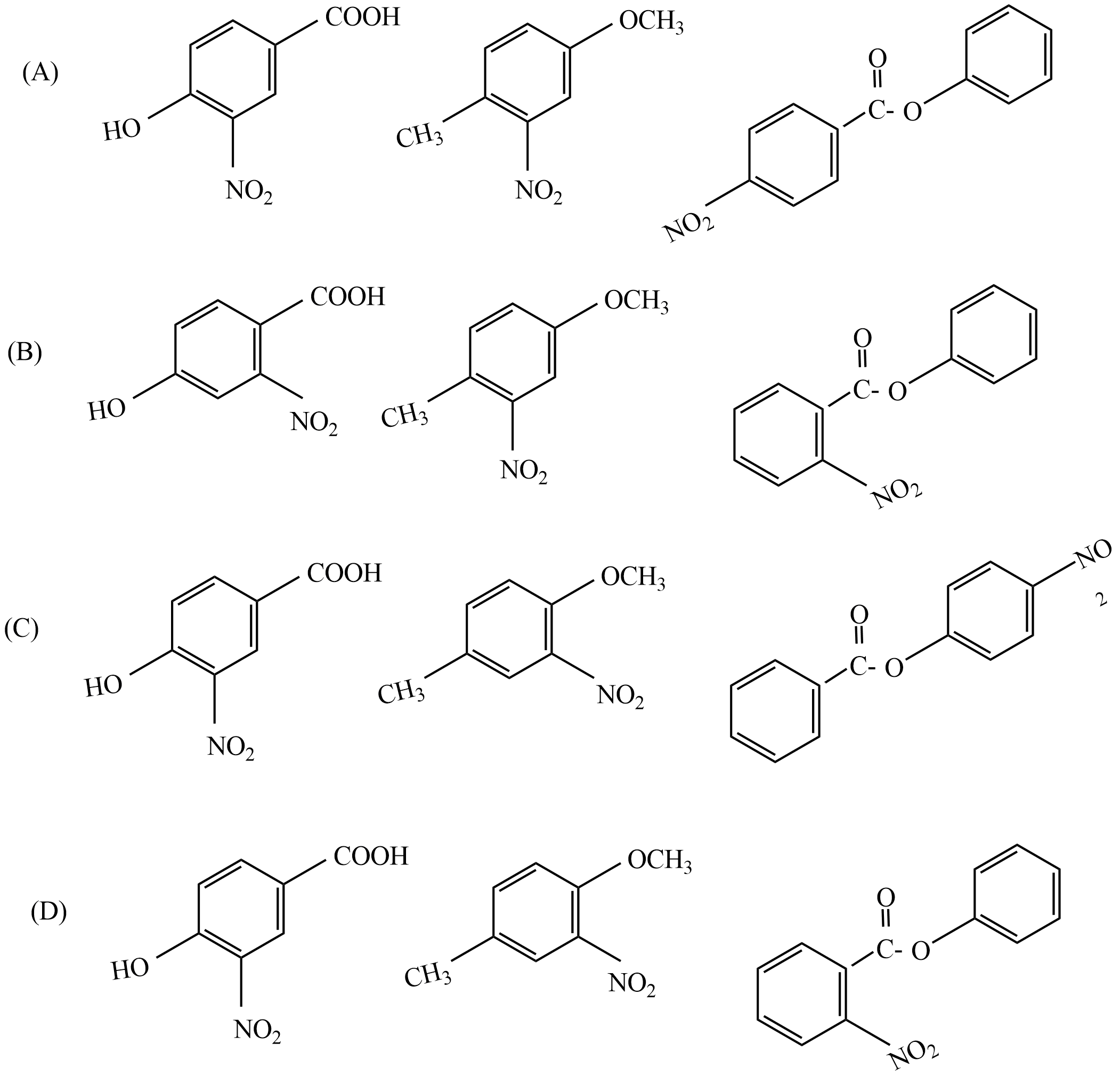
So, the correct answer is “Option C”.
Note: An atom that is attached to an aromatic ring is replaced with an electrophile in a reaction is an electrophilic aromatic substitution reaction. Aromatic nitrations, aromatic sulfonation, and Friedel-Craft reactions are examples of this type of reaction. The aromaticity of aromatic compounds play an important role in electrophilic aromatic substitution reactions.
Complete Solution :
Benzene ring involved in substitution reaction is two types based on the type of substituent that ring carries, which are activated rings and deactivated rings.
If the substituent on the ring which groups are donating electrons is an activated ring and group withdraws electrons is a deactivated ring.
Example of activating groups of decreasing order:
$-N{{H}_{2}} > -N{{R}_{2}} > -OH > -OR > -NHCOR > -C{{H}_{3}}$
Example of deactivating groups with relative order from highest to lowest deactivating groups:
$-N{{O}_{2}} > -C{{F}_{3}} > -COR > -CN > -COOR > -S{{O}_{3}}H$
The activating group in the benzene ring directs the reaction to the Ortho or Para position and the deactivating group in the benzene ring directs the reaction to the Meta position.
(i) When compound P reacted with $HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}$ the mixture, the major product formed is,
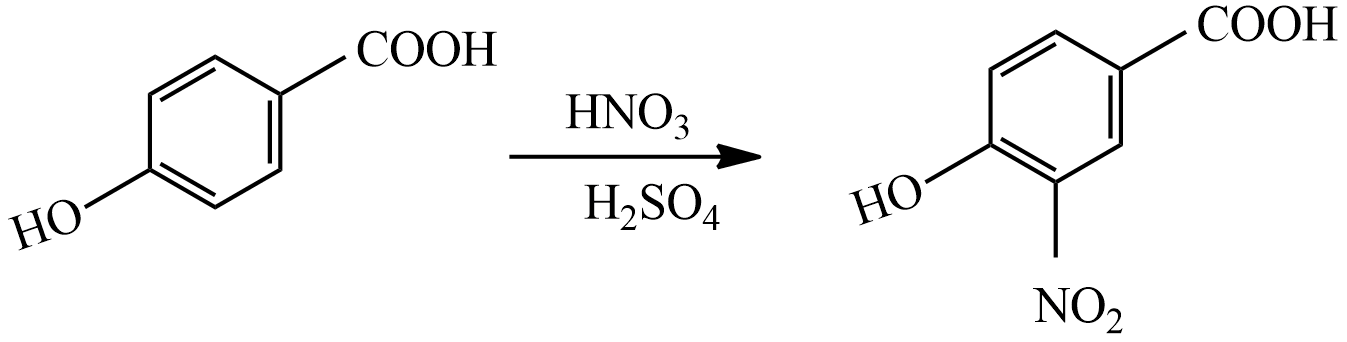
-OH group is an activating group and –COOH deactivating group, hence electrophile attack to the ortho position of the activating group –OH.
(ii) When compound Q reacted with $HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}$ the mixture, the major product formed is,
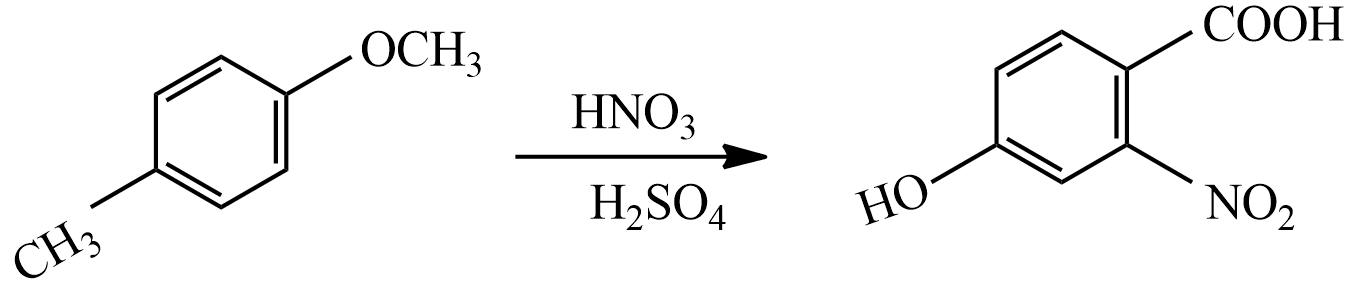
Both $-OC{{H}_{3}}$ and $-C{{H}_{3}}$ groups are activating groups which are directing Ortho or Para positions. But $-OC{{H}_{3}}$ group is a strong activating group than $-C{{H}_{3}}$ , so electrophile $N{{O}_{2}}^{-}$ attacked to Ortho position.
(iii) When compound S reacted with $HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}$ the mixture, the major product formed is,

The ring I, is deactivated and ring II is activated, hence electrophile attack at Para position of ring II which is a less hindered position.
So, the correct answer is “Option C”.
Note: An atom that is attached to an aromatic ring is replaced with an electrophile in a reaction is an electrophilic aromatic substitution reaction. Aromatic nitrations, aromatic sulfonation, and Friedel-Craft reactions are examples of this type of reaction. The aromaticity of aromatic compounds play an important role in electrophilic aromatic substitution reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




