
The compound ${{C}_{7}}{{H}_{8}}$ undergoes the following reactions:
${{C}_{7}}{{H}_{8}}\text{ }\xrightarrow{3C{{l}_{2}}/\Delta }\text{ }A\text{ }\xrightarrow{B{{r}_{2}}/Fe}\text{ }B\text{ }\xrightarrow{Zn/HCl}\text{ }C$. The product C is:
(A) 3 - bromo -2,4,6 - trichlorotoluene
(B) m - bromotoluene
(C) p - bromotoluene
(D) o - bromotoluene
Answer
588k+ views
Hint: Draw the expanded structure of the compound ${{C}_{7}}{{H}_{8}}$. Chlorine acts as a substituting group that will replace hydrogen atoms attached to the carbon atom. Bromine in the presence of iron generates an electrophile. Now zinc in the presence of HCl substitutes the chlorine atoms with hydrogen.
Complete step by step answer:
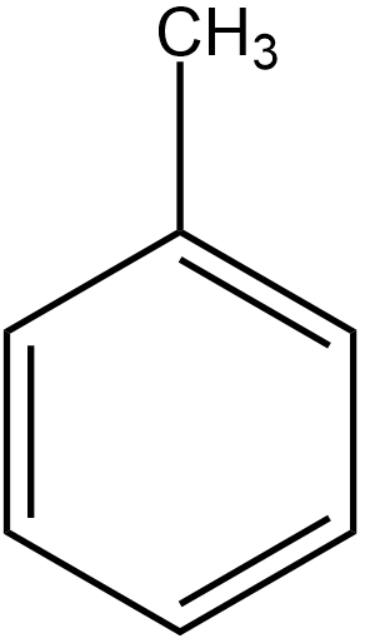
We will first draw the structure of ${{C}_{7}}{{H}_{8}}$. The degree of unsaturation present in the organic compounds is 4.
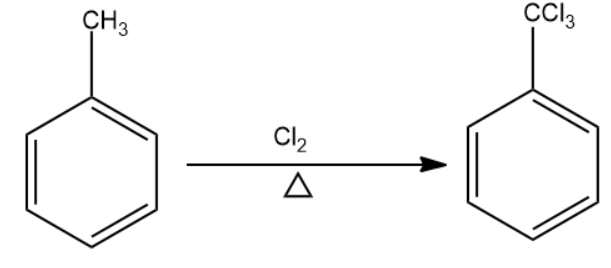
Chlorine in the presence of heat will replace the hydrogen atoms present in the methyl group to form methyl trichloride group. This becomes compound (A).
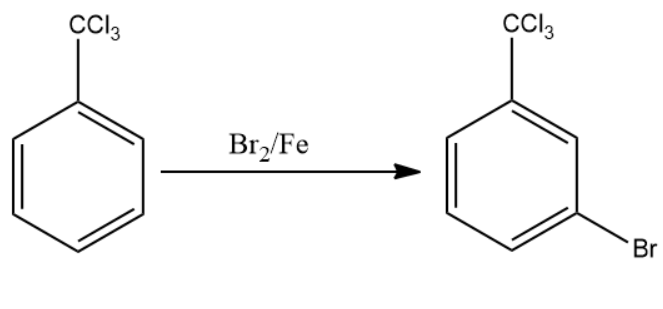
The compound formed reacts with the next reagent which is $B{{r}_{2}}/Fe$. The reagent forms a bromine electrophile which attaches at the meta position of the aromatic compound. This is compound
(B).
Once product (B) is formed, the compound is made to react with anhydrous zinc chloride in the presence of hydrochloric acid. This reagent replaces the chlorine atoms present with the external carbon atoms with the hydrogen atom from hydrochloric acid. This is compound (C).
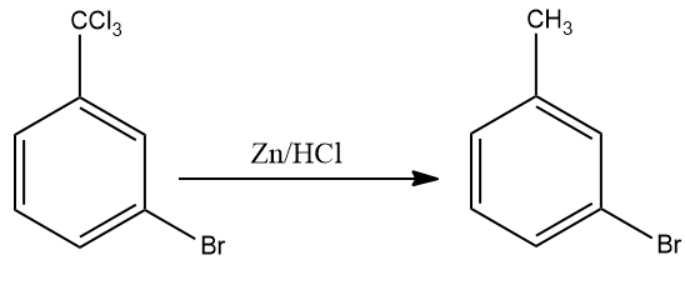
The final product formed i.e. compound (C) is m - bromotoluene.
So, the correct answer is “Option B”.
Note: It is important to know that the common name of an aromatic compound is also considered as the IUPAC name of the compound. For example, toluene is considered the IUPAC name along with methyl benzene for the same aromatic compound.
Complete step by step answer:
We will first draw the structure of ${{C}_{7}}{{H}_{8}}$. The degree of unsaturation present in the organic compounds is 4.

Chlorine in the presence of heat will replace the hydrogen atoms present in the methyl group to form methyl trichloride group. This becomes compound (A).

The compound formed reacts with the next reagent which is $B{{r}_{2}}/Fe$. The reagent forms a bromine electrophile which attaches at the meta position of the aromatic compound. This is compound
(B).

Once product (B) is formed, the compound is made to react with anhydrous zinc chloride in the presence of hydrochloric acid. This reagent replaces the chlorine atoms present with the external carbon atoms with the hydrogen atom from hydrochloric acid. This is compound (C).

The final product formed i.e. compound (C) is m - bromotoluene.
So, the correct answer is “Option B”.
Note: It is important to know that the common name of an aromatic compound is also considered as the IUPAC name of the compound. For example, toluene is considered the IUPAC name along with methyl benzene for the same aromatic compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




