
The bonds present in borazine/borazole are:
a.) $12\sigma $,$3\pi $
b.) $9\sigma $,$6\pi $
c.) $6\sigma $,$6\pi $
d.) $9\sigma $,$9\pi $
Answer
583.8k+ views
Hint: Bor in borazine indicates boron and azo indicates nitrogen . As seen in structure, borazole resembles benzene in structure and benzene has 12 sigma and 3 pi bonds. structure of borazole is
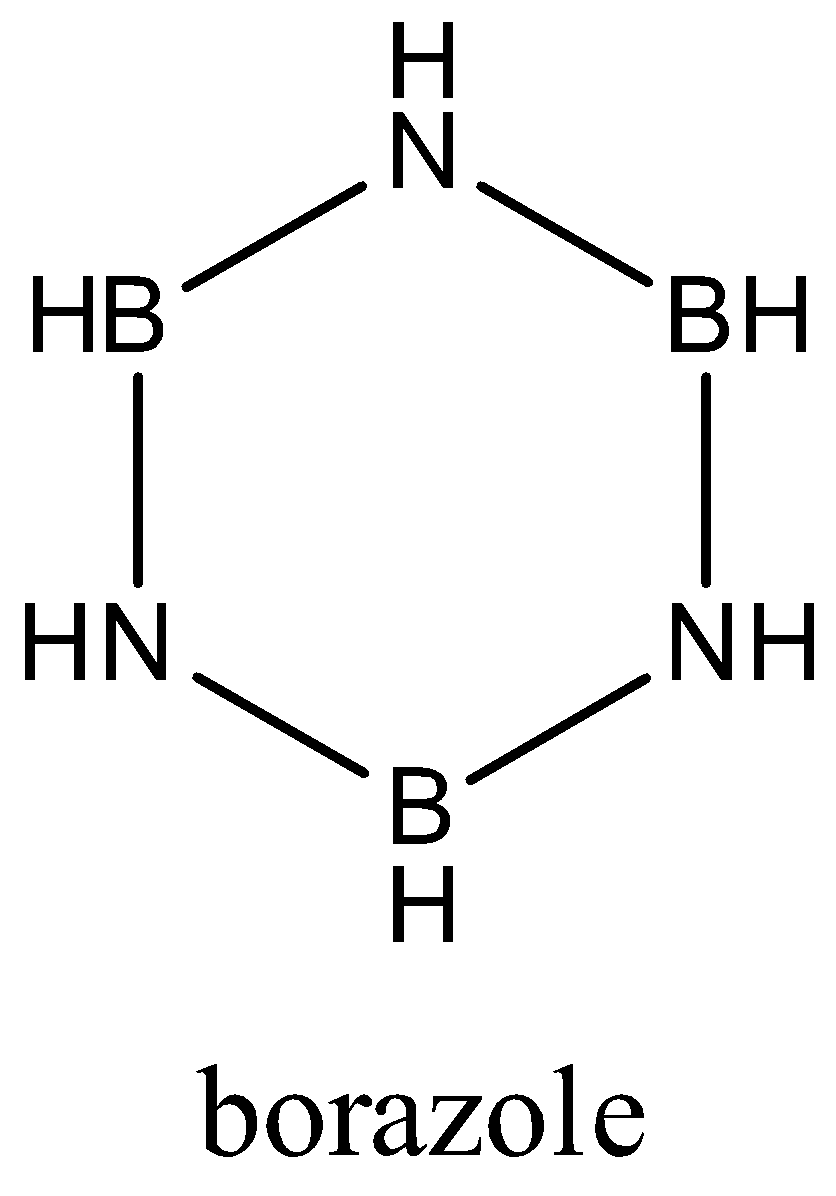
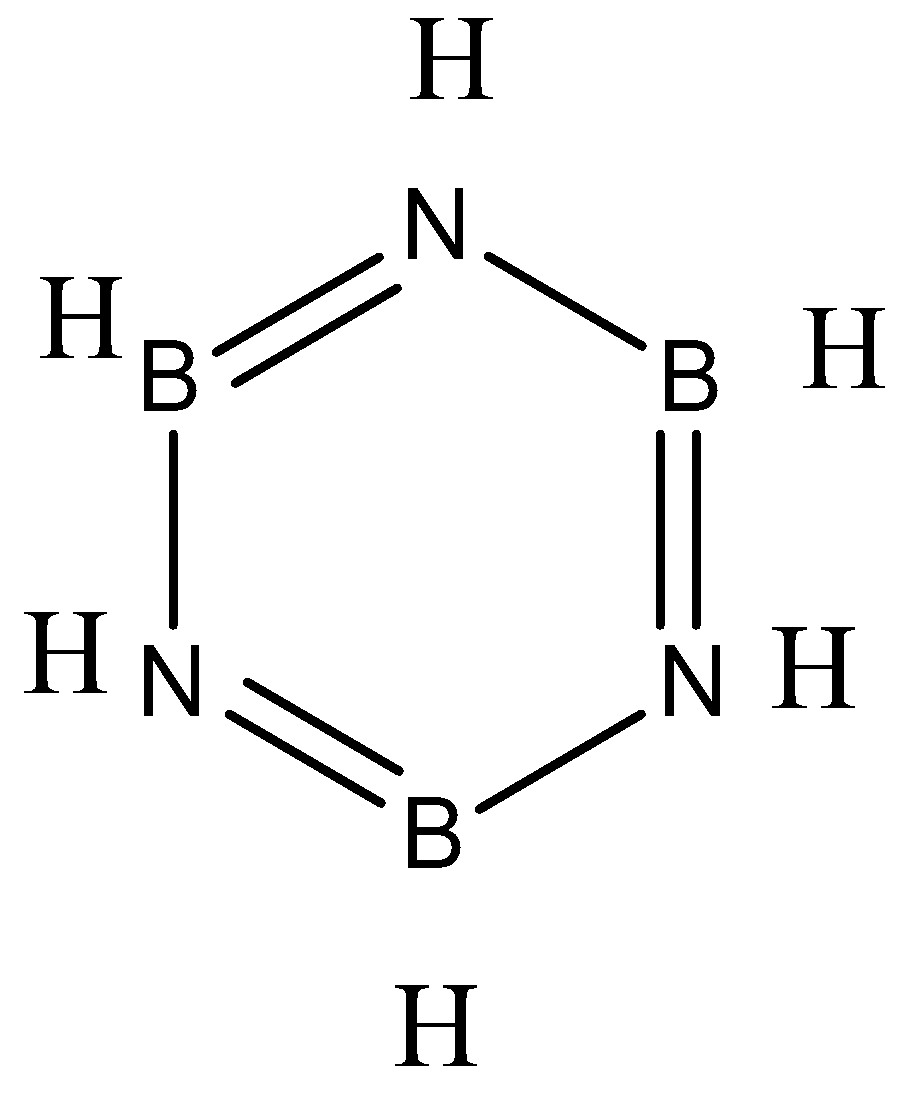
Complete step by step answer:
Borazole is also known as borazine, ${{B}_{3}}{{H}_{6}}{{N}_{3}}$ is chemical formula of borazole.
In borazine, three units of BH and three units of NH are arranged alternately.
Borazole has the same number of electrons and the same structure as benzene. As it has the same number of electrons, it is isoelectronic with benzene and has same structure as benzene, so it is isostructural. So, borazine is known as inorganic benzene.
When diborane is mixed with ammonia in ratio 1:2 at 250 to 300 degree Celsius, so borazine is synthesized.
More efficient method is when lithium borohydride is treated with ammonium chloride.
Borazine follows Huckle’s rule of aromaticity and as B-N bonds have equal lengths, it may be aromatic, so as structure suggests, there are 3 pi bonds, In each pi bond , there is one single bond and double bond and there are three B-H sigma bonds, three N-H sigma bonds, six B-N sigma bonds.
The bonds present in borazine are (A) $12\sigma $, $3\pi $.
Note: Boron acts as Lewis acid as it accepts lone pair of electrons and nitrogen acts as Lewis base as it donates lone pair of electrons. Electronegativity of boron is 2.04 and nitrogen has electronegativity as 3.04, so due to electronegativity difference between boron and nitrogen , there is unequal sharing of charge.


Complete step by step answer:
Borazole is also known as borazine, ${{B}_{3}}{{H}_{6}}{{N}_{3}}$ is chemical formula of borazole.
In borazine, three units of BH and three units of NH are arranged alternately.
Borazole has the same number of electrons and the same structure as benzene. As it has the same number of electrons, it is isoelectronic with benzene and has same structure as benzene, so it is isostructural. So, borazine is known as inorganic benzene.
When diborane is mixed with ammonia in ratio 1:2 at 250 to 300 degree Celsius, so borazine is synthesized.
More efficient method is when lithium borohydride is treated with ammonium chloride.
Borazine follows Huckle’s rule of aromaticity and as B-N bonds have equal lengths, it may be aromatic, so as structure suggests, there are 3 pi bonds, In each pi bond , there is one single bond and double bond and there are three B-H sigma bonds, three N-H sigma bonds, six B-N sigma bonds.
The bonds present in borazine are (A) $12\sigma $, $3\pi $.
Note: Boron acts as Lewis acid as it accepts lone pair of electrons and nitrogen acts as Lewis base as it donates lone pair of electrons. Electronegativity of boron is 2.04 and nitrogen has electronegativity as 3.04, so due to electronegativity difference between boron and nitrogen , there is unequal sharing of charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




