
The bond in the formation of fluorine molecule will be
A.due to \[s - s\] overlapping
B. due to \[s - p\] overlapping
C. due to \[p - p\] overlapping
D. due to \[d - d\] overlapping
Answer
501.9k+ views
Hint: The bond will be formed according to valence orbital theory. The valence shell of both fluorine atoms will overlap each other. Since the overlapping of atomic orbitals takes place, the bond formed will be a sigma bond. The orbitals which take part in overlapping are the outermost orbital of the atom.
Complete answer:
The fluorine molecule consists of two fluorine atoms which are bonded with sigma bond. The bond formation takes place according to valence bond theory. Since we know that the atomic number of fluorine is nine. Therefore its electronic configuration can be written as \[1{s^2},2{s^2},2{p^4}\] , hence it has a vacant \[p - \]orbital. The valence shell electronic configuration of the valence shell can be written as
\[2{s^2},{\text{ }}2{p_x}^2,{\text{ }}2{p_y}^2,{\text{ }}2{p_z}^1\]. Here we can observe that \[2{p_z}\] contains only one electron. Thus this orbital will take part in bond formation. The overlapping of atomic orbital can be shown as,
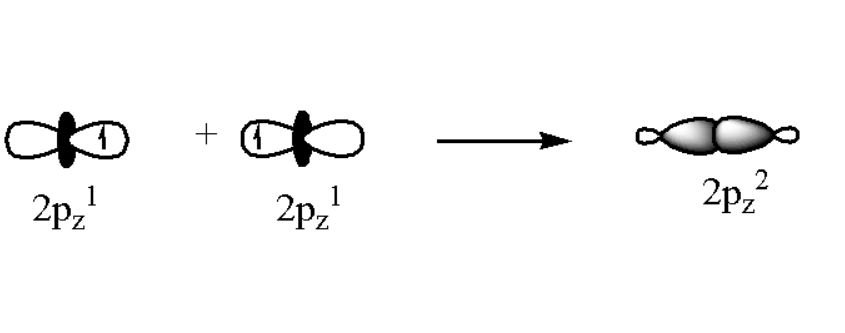
Thus we observe that two electrons from each fluorine atoms makes a bond and overlapping takes place. As a result an orbital which contains two electron is formed. This is called as \[p - p\] overlapping. Hence the correct answer is option C. due to \[p - p\] overlapping.
Note:
This overlapping of atomic orbital is called end to end overlapping which results in formation of sigma bonds between two atoms. The direction of rotation of electrons is opposite to one another. Two orbitals overlap to generate new degenerate orbitals, which is the basic rule of valence bond theory. The bond which is formed in this overlapping is a covalent bond.
Complete answer:
The fluorine molecule consists of two fluorine atoms which are bonded with sigma bond. The bond formation takes place according to valence bond theory. Since we know that the atomic number of fluorine is nine. Therefore its electronic configuration can be written as \[1{s^2},2{s^2},2{p^4}\] , hence it has a vacant \[p - \]orbital. The valence shell electronic configuration of the valence shell can be written as
\[2{s^2},{\text{ }}2{p_x}^2,{\text{ }}2{p_y}^2,{\text{ }}2{p_z}^1\]. Here we can observe that \[2{p_z}\] contains only one electron. Thus this orbital will take part in bond formation. The overlapping of atomic orbital can be shown as,

Thus we observe that two electrons from each fluorine atoms makes a bond and overlapping takes place. As a result an orbital which contains two electron is formed. This is called as \[p - p\] overlapping. Hence the correct answer is option C. due to \[p - p\] overlapping.
Note:
This overlapping of atomic orbital is called end to end overlapping which results in formation of sigma bonds between two atoms. The direction of rotation of electrons is opposite to one another. Two orbitals overlap to generate new degenerate orbitals, which is the basic rule of valence bond theory. The bond which is formed in this overlapping is a covalent bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




