
The best metal to be used for photoemission is:
A.Potassium
B.Sodium
C.Cesium
D.Lithium
Answer
578.4k+ views
Hint: Basically, the process through which photoelectrons are ejected from the surface of the metal due to the action of light is commonly known as photoemission. Moreover, for this effect to occur, the photons that are incident on the surface of the metal must carry sufficient energy to overcome the attractive forces that bind the electrons to the nuclei of the metals.
Complete step by step answer:
Basically, the photoelectric effect is a phenomenon in which the electrons are ejected from the surface of a metal when light is incident on it. These ejected electrons are known as photoelectrons and the process through which photoelectrons are ejected from the surface of the metal due to the action of light is commonly referred as photoemission.
Now, this phenomenon occurs because the electrons at the surface of the metal tend to absorb energy from the incident light and use it to overcome the attractive forces that bind them to the metallic nuclei. An illustration is as shown:
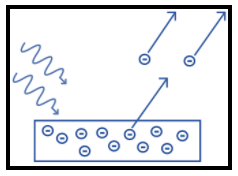
Now, photoelectric effect occurs easily if the metal has low ionization potential. So, among the given options, cesium has the lowest ionization potential and hence it is best suitable for photoelectric effect. It is a soft, silvery-gold alkali element that is quickly attacked by air and reacts explosively with water. It occurs in the environment mainly due to erosion and weathering of rocks.
Hence, option C is correct.
Note: Cesium has various applications. It is used in thermionic generators which convert heat energy into electrical energy. It is also used in the manufacturing of optical instruments and is used to remove oxygen from light bulbs and vacuum tubes.
Complete step by step answer:
Basically, the photoelectric effect is a phenomenon in which the electrons are ejected from the surface of a metal when light is incident on it. These ejected electrons are known as photoelectrons and the process through which photoelectrons are ejected from the surface of the metal due to the action of light is commonly referred as photoemission.
Now, this phenomenon occurs because the electrons at the surface of the metal tend to absorb energy from the incident light and use it to overcome the attractive forces that bind them to the metallic nuclei. An illustration is as shown:

Now, photoelectric effect occurs easily if the metal has low ionization potential. So, among the given options, cesium has the lowest ionization potential and hence it is best suitable for photoelectric effect. It is a soft, silvery-gold alkali element that is quickly attacked by air and reacts explosively with water. It occurs in the environment mainly due to erosion and weathering of rocks.
Hence, option C is correct.
Note: Cesium has various applications. It is used in thermionic generators which convert heat energy into electrical energy. It is also used in the manufacturing of optical instruments and is used to remove oxygen from light bulbs and vacuum tubes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




