
The average oxidation state of chlorine in bleaching powder is:
A. -1
B. +1
C. Zero
D. -2 as well as +2
Answer
594k+ views
Hint: We know that the molecular formula of bleaching powder is $Ca(OCl)Cl$. In the molecular structure of bleaching powder, $Ca$is the centred atom with $OCl$ and $Cl$ ions being branched to it.
Complete answer:
The structure of $C{{a}^{2+}}{{(OCl)}^{-}}C{{l}^{-}}$ is as follows:
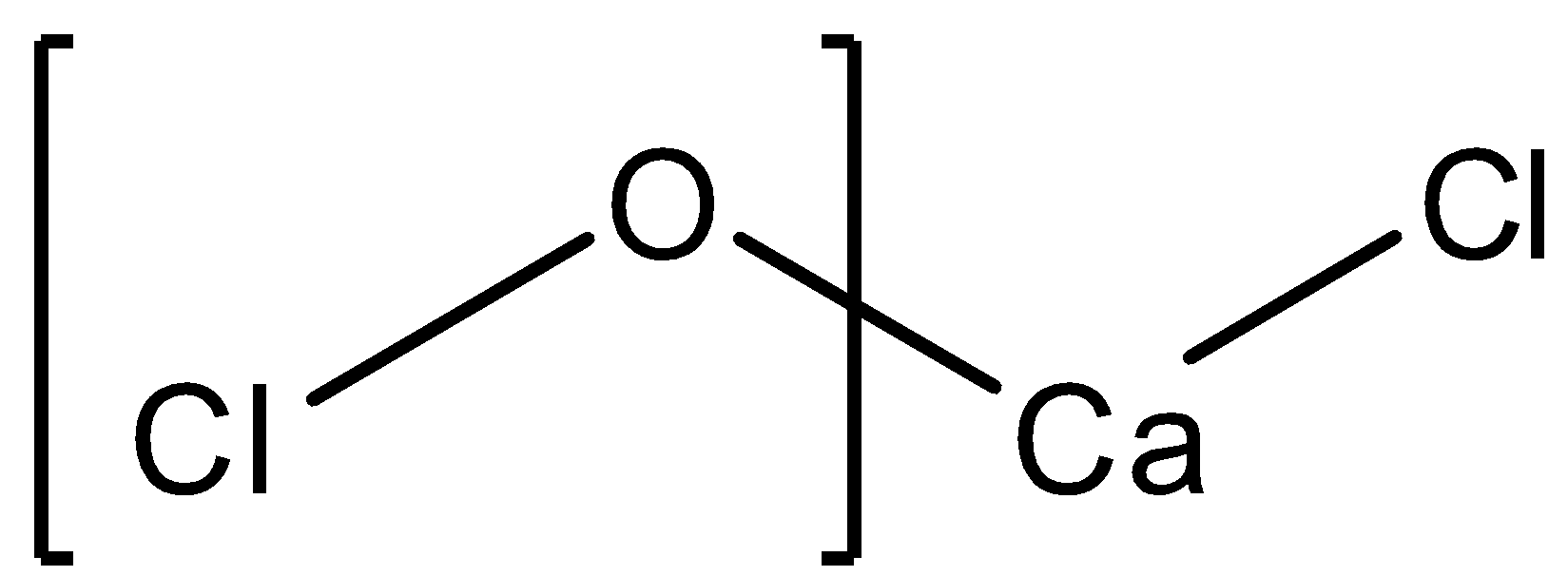
We can see that calcium having a valency of +2, gains electrons from chlorine and oxygen. While oxygen having a valency of -2 shares an electron with the other chlorine. This proves that the chlorine atom attached directly to calcium has an oxidation state of -1. Calcium itself has an oxidation state of +2 which brings the following equation stating the oxidation state of $OCl$ to be -1. The sum of the oxidation states of all the atoms is 0 since the molecule is neutral.
$\begin{align}
& +2\ +\ (-1)\ +\ (-1)\ =\text{ }\ 0 \\
& Ca\text{ }Cl\text{ }OCl\text{ }Stability \\
\end{align}$
In the above equation, we see that stability is reached by the $Ca(OCl)Cl$ molecule when the oxidation state of $OCl$ is -1. We know that the oxidation state of oxygen is -2. Hence, to bring the oxidation state of $OCl$ as -1, the Chlorine atom attached to Oxygen must have an oxidation state of +1 as calculated from the following equation.
$(-2)\ +\ x=(-1)$
Where, x is the oxidation state of chlorine atom attached to oxygen.
From the above equation, we get the oxidation state of chlorine attached to oxygen to be +1.
Therefore, oxidation states of $Cl=+1,-1$
Hence, average oxidation state of$Cl=\text{ }\dfrac{(+1)+(-1)}{2}\text{ = }\dfrac{0}{2}\text{ = 0}$
The average oxidation state of $Cl$ atom in $Ca(OCl)Cl$ is zero.
Therefore, the correct answer is Option C.
Note: We should be knowing that bleaching powder is yellow in colour, having a strong smell of chlorine. It is soluble in water but aqueous solution is never found due to the presence of impurities. It loses chlorine by the action of carbon dioxide.
Although the usual oxidation state of chlorine is -1, since oxygen is more electronegative than chlorine, it adapts to the +1 oxidation state.
Complete answer:
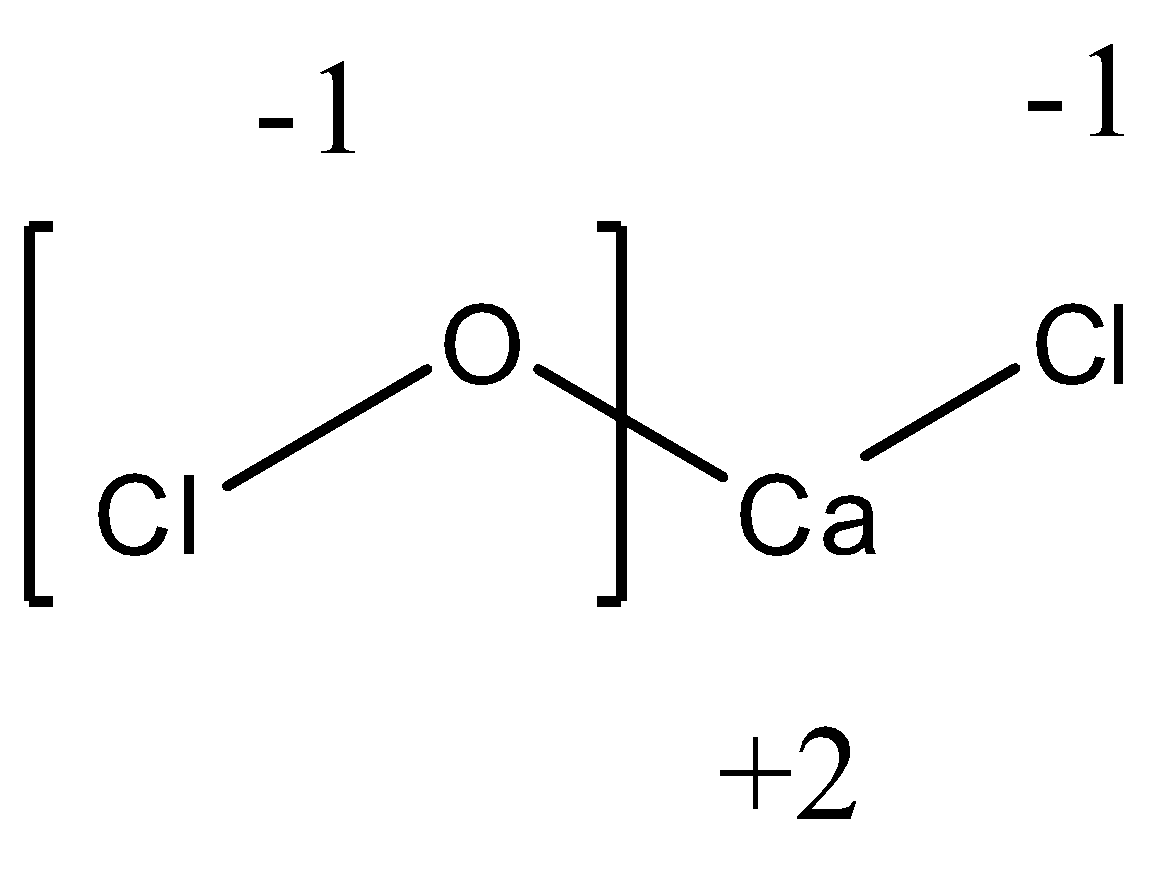
The structure of $C{{a}^{2+}}{{(OCl)}^{-}}C{{l}^{-}}$ is as follows:

We can see that calcium having a valency of +2, gains electrons from chlorine and oxygen. While oxygen having a valency of -2 shares an electron with the other chlorine. This proves that the chlorine atom attached directly to calcium has an oxidation state of -1. Calcium itself has an oxidation state of +2 which brings the following equation stating the oxidation state of $OCl$ to be -1. The sum of the oxidation states of all the atoms is 0 since the molecule is neutral.
$\begin{align}
& +2\ +\ (-1)\ +\ (-1)\ =\text{ }\ 0 \\
& Ca\text{ }Cl\text{ }OCl\text{ }Stability \\
\end{align}$

In the above equation, we see that stability is reached by the $Ca(OCl)Cl$ molecule when the oxidation state of $OCl$ is -1. We know that the oxidation state of oxygen is -2. Hence, to bring the oxidation state of $OCl$ as -1, the Chlorine atom attached to Oxygen must have an oxidation state of +1 as calculated from the following equation.
$(-2)\ +\ x=(-1)$
Where, x is the oxidation state of chlorine atom attached to oxygen.
From the above equation, we get the oxidation state of chlorine attached to oxygen to be +1.
Therefore, oxidation states of $Cl=+1,-1$
Hence, average oxidation state of$Cl=\text{ }\dfrac{(+1)+(-1)}{2}\text{ = }\dfrac{0}{2}\text{ = 0}$
The average oxidation state of $Cl$ atom in $Ca(OCl)Cl$ is zero.
Therefore, the correct answer is Option C.
Note: We should be knowing that bleaching powder is yellow in colour, having a strong smell of chlorine. It is soluble in water but aqueous solution is never found due to the presence of impurities. It loses chlorine by the action of carbon dioxide.
Although the usual oxidation state of chlorine is -1, since oxygen is more electronegative than chlorine, it adapts to the +1 oxidation state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




