
The artificial sweetener containing chlorine that has the appearance and taste as that of sugar and is stable at cooking temperature is-
A) Aspartame
B) Saccharin
C) Sucralose
D) Alitame
Answer
576.6k+ views
Hint: Alitame and Sucralose are the only artificial sweeteners that are stable at cooking temperatures.
Complete step by step answer:
The artificial sweetener containing chlorine that has the appearance and taste as that of sugar and is stable at cooking temperature is Sucralose. Its chemical formula is\[{{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{19}}}}{\text{C}}{{\text{l}}_{\text{3}}}{{\text{O}}_{\text{8}}}\] .Its structure is-
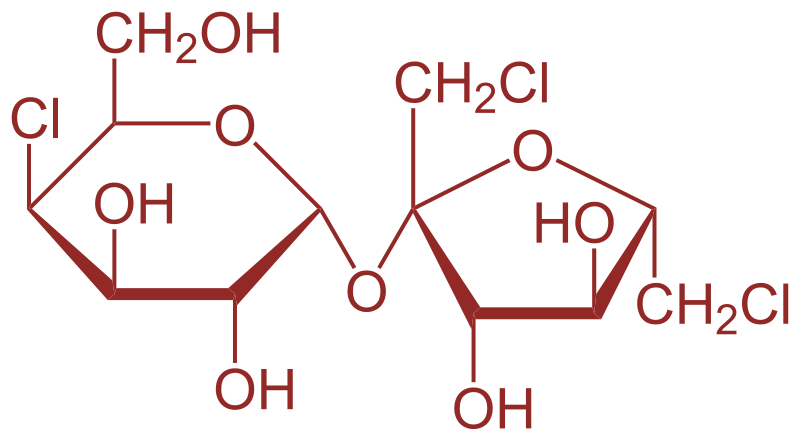
It is derived from sugar through a chemical process in which $3$ hydroxyl groups are replaced by $3$ chlorine atoms on sugar molecules which produces a sweetener which has no calories and is $600$ times sweeter than sucrose. In Sucralose, ${\text{4 - chloro - 4 - deoxy - alpha - D - galactopyranose}}$ and ${\text{1,6 - dichloro - 1,6 - dideoxy - beta - D - fructofuranose}}$ are the units which are linked by glycosidic bond.
The properties of Sucralose are-
-It is a disaccharide derivative and since it has chlorine in its formula it is also known as organo-chlorine compound.
-It is a white crystalline powder and has a sweet taste.
-It has a melting point of ${130^ \circ }{\text{C}}$.
-It is soluble in water, methanol and ethanol but only slightly soluble in ethyl acetate.
-It also plays a role as an environmental contaminant.
Hence, the correct answer is ‘C’.
Note:
Aspartame and saccharin decompose at cooking temperature hence they are not used in baked foods. Alitame does not contain chlorine so only Sucralose is the possible answer. The uses of Sucralose are –
-It is used as a food additive and flavor enhancer.
-It is also a xenobiotic agent.
Complete step by step answer:
The artificial sweetener containing chlorine that has the appearance and taste as that of sugar and is stable at cooking temperature is Sucralose. Its chemical formula is\[{{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{19}}}}{\text{C}}{{\text{l}}_{\text{3}}}{{\text{O}}_{\text{8}}}\] .Its structure is-

It is derived from sugar through a chemical process in which $3$ hydroxyl groups are replaced by $3$ chlorine atoms on sugar molecules which produces a sweetener which has no calories and is $600$ times sweeter than sucrose. In Sucralose, ${\text{4 - chloro - 4 - deoxy - alpha - D - galactopyranose}}$ and ${\text{1,6 - dichloro - 1,6 - dideoxy - beta - D - fructofuranose}}$ are the units which are linked by glycosidic bond.
The properties of Sucralose are-
-It is a disaccharide derivative and since it has chlorine in its formula it is also known as organo-chlorine compound.
-It is a white crystalline powder and has a sweet taste.
-It has a melting point of ${130^ \circ }{\text{C}}$.
-It is soluble in water, methanol and ethanol but only slightly soluble in ethyl acetate.
-It also plays a role as an environmental contaminant.
Hence, the correct answer is ‘C’.
Note:
Aspartame and saccharin decompose at cooking temperature hence they are not used in baked foods. Alitame does not contain chlorine so only Sucralose is the possible answer. The uses of Sucralose are –
-It is used as a food additive and flavor enhancer.
-It is also a xenobiotic agent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




