
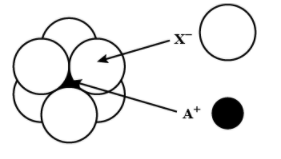
The arrangement of ${{X}^{-}}$ around ${{A}^{+}}$ ion in solid AX is given in the figure (not drawn to scale). If the radius of ${{X}^{-}}$ is 250 pm, the radius (in pm) of ${{A}^{+}}$ will be:
(A) 104
(B) 125
(C) 183
(D) 57

Answer
577.5k+ views
Hint: The octahedral voids are located at the body center and at the center of the 12 edges of the cube. The number of octahedral voids per unit cell varies for different types of arrangements.
Complete step by step solution:
According to the given figure the ${{A}^{+}}$ ion is present in the octahedral void of the ${{X}^{-}}$ ion.
-Given in the question:
-The radius of the ${{X}^{-}}$ is = 250pm
-To solve this question we should know the relation between the limiting radius of the octahedral void to the radius of the sphere
-The relation between the limiting radius of the octahedral void to the radius of the sphere is:
-Radius of the octahedral void = 0.414 radius of the sphere
\[{{r}_{void}}=0.414{{r}_{sphere}}\]
-Radius of void = $0.414X250$ = 103.5 pm
-103.5 is approximately equal to 104
Hence the correct answer is option (A) i.e. 104 pm
Additional information:
Tetrahedral void- in the cubic close packing, sphere of the second layer lie above the triangular void of the first layer. Each sphere touches the three spheres of the first layer. If we join the center of these four spheres it forms a tetrahedron and the empty space left over by joining the center of these spheres, it forms a tetrahedral void.
Note: Pm stands for Pico meter, it is a unit of length in the metric system. 1 Picometer is equal to ${{10}^{-12}}$ meter. The Pico meter is one thousand of the nanometer, one trillionth of the meter and one millionth of a micrometer.
Complete step by step solution:
According to the given figure the ${{A}^{+}}$ ion is present in the octahedral void of the ${{X}^{-}}$ ion.
-Given in the question:
-The radius of the ${{X}^{-}}$ is = 250pm
-To solve this question we should know the relation between the limiting radius of the octahedral void to the radius of the sphere
-The relation between the limiting radius of the octahedral void to the radius of the sphere is:
-Radius of the octahedral void = 0.414 radius of the sphere
\[{{r}_{void}}=0.414{{r}_{sphere}}\]
-Radius of void = $0.414X250$ = 103.5 pm
-103.5 is approximately equal to 104
Hence the correct answer is option (A) i.e. 104 pm
Additional information:
Tetrahedral void- in the cubic close packing, sphere of the second layer lie above the triangular void of the first layer. Each sphere touches the three spheres of the first layer. If we join the center of these four spheres it forms a tetrahedron and the empty space left over by joining the center of these spheres, it forms a tetrahedral void.
Note: Pm stands for Pico meter, it is a unit of length in the metric system. 1 Picometer is equal to ${{10}^{-12}}$ meter. The Pico meter is one thousand of the nanometer, one trillionth of the meter and one millionth of a micrometer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




