
The acid present in the body of red ant is ______.
Answer
610.2k+ views
Hint: An ant is called ‘Formica’ in Latin. The acid found in red ants is the simplest carboxylic acid.
Complete step by step answer:
The acid found in the body of red ant is formic acid, named after the Latin name of an ant ‘Formica’. The IUPAC name of formic acid is methanoic acid. Methanoic acid (HCOOH) is the simplest carboxylic acid. The irritating itching sensation after an ant’s bite is thus due to formic acid. Formic acid is dangerous to the human body at high concentrations, but very useful at low concentrations. It is used as a food preservative due to its antibacterial nature. It is also used in pesticides and cosmetic additives. It is also used to help a variety of industrial processes to occur. Methanoic acid consists of a carbon doubly bonded to an oxygen and a single bond joining it to a hydroxyl (OH) group. The structure of formic acid is:
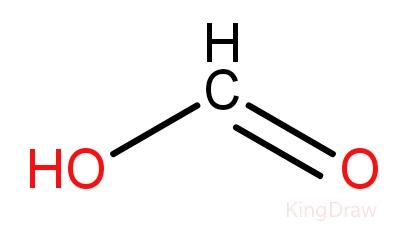
Additional information: Our bodies also make small amounts of formic acid from the methanol we intake. Some of the methanol is also produced in our body from aspartame. Our body converts aspartame into aspartic acid, methanol and phenylalanine. The methanol is therefore converted into formic acid.
Note: One can think formic acid to be very corrosive to skin. But formic acid is a mild naturally occurring acid. In small amounts, it is in fact very beneficial to the human body.
Complete step by step answer:
The acid found in the body of red ant is formic acid, named after the Latin name of an ant ‘Formica’. The IUPAC name of formic acid is methanoic acid. Methanoic acid (HCOOH) is the simplest carboxylic acid. The irritating itching sensation after an ant’s bite is thus due to formic acid. Formic acid is dangerous to the human body at high concentrations, but very useful at low concentrations. It is used as a food preservative due to its antibacterial nature. It is also used in pesticides and cosmetic additives. It is also used to help a variety of industrial processes to occur. Methanoic acid consists of a carbon doubly bonded to an oxygen and a single bond joining it to a hydroxyl (OH) group. The structure of formic acid is:

Additional information: Our bodies also make small amounts of formic acid from the methanol we intake. Some of the methanol is also produced in our body from aspartame. Our body converts aspartame into aspartic acid, methanol and phenylalanine. The methanol is therefore converted into formic acid.
Note: One can think formic acid to be very corrosive to skin. But formic acid is a mild naturally occurring acid. In small amounts, it is in fact very beneficial to the human body.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




