
The ${}_{98}^{250}Cf$ is split into two parts of almost equal mass. What is the order of magnitude for potential energy of interaction of the two parts when they are just touching each other? (in ${10^n}{\text{ }}eV$). Find value of $n$.
Answer
493.2k+ views
Hint: First of all, we have to find the atomic mass of each subdivided atom.The two parts that are being split are nucleus. Then we have to find the radius of each nucleus. Then by the help of the formula to find the potential energy, we will find the answer.
Formula used:
The formula to find the potential energy between the two nucleus, when they are in touch with each other is $ - \dfrac{{GMm}}{r} - - - - - \left( 1 \right)$ where, $G = $ Universal Gravitational Constant, $M = m = $ mass of each nucleus, $r = $ distance between the centre of each nucleus.
Complete step by step answer:
Let us first consider the splitting of the atom. Let the subdivided parts be $X$.Thus, we get,
${}_{98}^{250}Cf \to {}_{49}^{125}X + {}_{49}^{125}X$
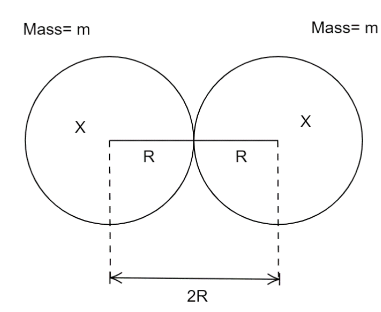
Let the radius of each nucleus be $R$.
$R = {R_0}{A^{\dfrac{1}{3}}}$
where ${R_0} = 1.25 \times {10^{ - 15}}{\text{ }}m$ is constant and $A = $ atomic number.
$R = 1.25 \times {10^{ - 15}} \times {(125)^{\dfrac{1}{3}}}$
$ \Rightarrow R = 6.25 \times {10^{ - 15}}{\text{ }}m$
The formula to find the potential energy between the two nucleus, when they are in touch with each other is $ - \dfrac{{GMm}}{r} - - - - - \left( 1 \right)$ where, $G = $ Universal Gravitational Constant, $M = m = $ mass of each nucleus, $r = $ distance between the centre of each nucleus.
The values of the given variables are,
$G = 6.67 \times {10^{ - 11}}{\text{ }}\dfrac{N}{{k{g^2}{m^2}}}$
$\Rightarrow M = m = 125 \times 1.67 \times {10^{ - 27}}{\text{ }}kg$
$\Rightarrow r = 2R = 2 \times 6.25 \times {10^{ - 15}}{\text{ }}m$
Substituting all the value sin equation $\left( 1 \right)$ we get,
$\text{Potential Energy} = - \dfrac{{6.67 \times {{10}^{ - 11}} \times {{(125 \times 1.67 \times {{10}^{ - 27}})}^2}}}{{2 \times 6.25 \times {{10}^{ - 15}}}}{\text{ }}J$
$\Rightarrow \text{Potential Energy} \approx \dfrac{{2.9 \times {{10}^{ - 60}}}}{{12.5 \times {{10}^{ - 15}}}} \\
\Rightarrow \text{Potential Energy}\approx 2.3 \times {10^{ - 46}}{\text{ }}J$
In terms of electron Volt or $eV$ we get,
$\text{Potential Energy}= \dfrac{{2.3 \times {{10}^{ - 46}}}}{{1.6 \times {{10}^{ - 19}}}} \\
\therefore \text{Potential Energy}= 1.4 \times {10^{ - 27}}{\text{ }}eV$
Thus, the order of $n = - 27$.
Note: It must be noted that the potential energy of a system between two charges is the same for two atoms. The mass of each nucleus is found from the atomic mass of it to the mass of each atom. In order to find anything in electron volt we must divide the following variable with the amount of charge associated with each electron.
Formula used:
The formula to find the potential energy between the two nucleus, when they are in touch with each other is $ - \dfrac{{GMm}}{r} - - - - - \left( 1 \right)$ where, $G = $ Universal Gravitational Constant, $M = m = $ mass of each nucleus, $r = $ distance between the centre of each nucleus.
Complete step by step answer:
Let us first consider the splitting of the atom. Let the subdivided parts be $X$.Thus, we get,
${}_{98}^{250}Cf \to {}_{49}^{125}X + {}_{49}^{125}X$

Let the radius of each nucleus be $R$.
$R = {R_0}{A^{\dfrac{1}{3}}}$
where ${R_0} = 1.25 \times {10^{ - 15}}{\text{ }}m$ is constant and $A = $ atomic number.
$R = 1.25 \times {10^{ - 15}} \times {(125)^{\dfrac{1}{3}}}$
$ \Rightarrow R = 6.25 \times {10^{ - 15}}{\text{ }}m$
The formula to find the potential energy between the two nucleus, when they are in touch with each other is $ - \dfrac{{GMm}}{r} - - - - - \left( 1 \right)$ where, $G = $ Universal Gravitational Constant, $M = m = $ mass of each nucleus, $r = $ distance between the centre of each nucleus.
The values of the given variables are,
$G = 6.67 \times {10^{ - 11}}{\text{ }}\dfrac{N}{{k{g^2}{m^2}}}$
$\Rightarrow M = m = 125 \times 1.67 \times {10^{ - 27}}{\text{ }}kg$
$\Rightarrow r = 2R = 2 \times 6.25 \times {10^{ - 15}}{\text{ }}m$
Substituting all the value sin equation $\left( 1 \right)$ we get,
$\text{Potential Energy} = - \dfrac{{6.67 \times {{10}^{ - 11}} \times {{(125 \times 1.67 \times {{10}^{ - 27}})}^2}}}{{2 \times 6.25 \times {{10}^{ - 15}}}}{\text{ }}J$
$\Rightarrow \text{Potential Energy} \approx \dfrac{{2.9 \times {{10}^{ - 60}}}}{{12.5 \times {{10}^{ - 15}}}} \\
\Rightarrow \text{Potential Energy}\approx 2.3 \times {10^{ - 46}}{\text{ }}J$
In terms of electron Volt or $eV$ we get,
$\text{Potential Energy}= \dfrac{{2.3 \times {{10}^{ - 46}}}}{{1.6 \times {{10}^{ - 19}}}} \\
\therefore \text{Potential Energy}= 1.4 \times {10^{ - 27}}{\text{ }}eV$
Thus, the order of $n = - 27$.
Note: It must be noted that the potential energy of a system between two charges is the same for two atoms. The mass of each nucleus is found from the atomic mass of it to the mass of each atom. In order to find anything in electron volt we must divide the following variable with the amount of charge associated with each electron.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




