
Suggest a reason as to why CO is poisonous.
Answer
591k+ views
Hint: Carbon monoxide (CO) is a strong field ligand and forms stable complexes. Haemoglobin in blood is responsible for oxygen transport in the human body. CO has the ability to block the supply of oxygen to organs and tissues.
Complete step by step answer:
Haemoglobin present in red blood corpuscles (RBC) is a biochemical that plays an important role in supplying oxygen to different parts of our body.
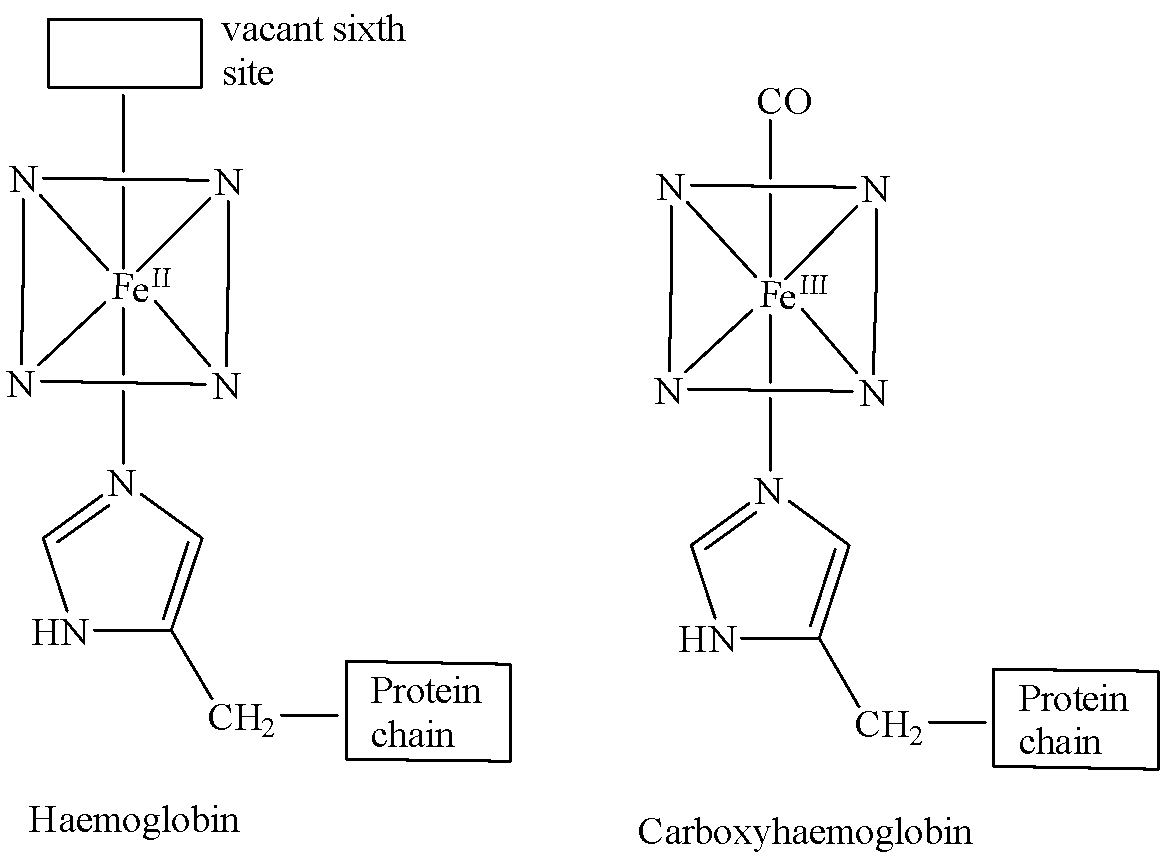
The structure of haemoglobin comprises four heme groups and polypeptide chains. Each heme group has an iron atom which has six coordination sites. Fe (II) is coordinated to four nitrogen atoms of a porphyrin ring. The fifth coordination site is occupied by the N-atom of histidine (an amino acid) group in the polypeptide chain. The sixth site remains vacant.
Haemoglobin picks up oxygen from the lungs and carries it to the tissues via the circulatory system. The sixth vacant position is then occupied by dioxygen (${{O}_{2}}$) to form an oxyhaemoglobin complex.
\[Hb+{{O}_{2}}\rightleftarrows Hb{{O}_{2}}\]
But we know that CO is a stronger ligand than${{O}_{2}}$. So, if CO is present in the surrounding air in excess, it combines with haemoglobin by linking to the Fe (II) at the sixth coordination site. The complex thus formed is called carboxyhemoglobin and it is 300 times more stable than oxyhaemoglobin.
\[Hb+CO\rightleftarrows HbCO\]
Thus, the supply of oxygen of oxygen to different tissues is prevented due to linkage of CO with haemoglobin. When the concentration of carboxyhemoglobin reaches about 3-4% in blood, the ability of haemoglobin to bind with oxygen is largely reduced.
The deficiency of oxygen results in difficulty in breathing, headache, dizziness, in certain cases where the concentration of CO in the surrounding air is more than 750 ppm, it leads to acute oxygen starvation, i.e. anoxia and results in coma and death.
Thus, the reason for the poisonous nature of CO is its ability to form a stable complex with haemoglobin.
Additional Information:
The maximum concentration of CO in the surrounding air should be 40 ppm for an exposure of 6-8 hours. Concentration of CO in the body of smokers is more than 5% and they are highly prone to acute oxygen starvation.
CO is produced from incomplete combustion of carbon. Patients suffering from CO poisoning are kept in high pressure oxygen containing chambers. The pressure of oxygen in those chambers is about 2.5 atm and thus, CO in haemoglobin is replaced by ${{O}_{2}}$.
\[HbCO+{{O}_{2}}\rightleftarrows Hb{{O}_{2}}+CO\]
Note:Under normal conditions, ${{O}_{2}}$ binds with haemoglobin to form oxyhaemoglobin. But, if the concentration of CO is more in the surrounding air then, haemoglobin forms complexes with CO and not ${{O}_{2}}$. This is because CO combines with haemoglobin more easily (about 200 times faster) than ${{O}_{2}}$ to form a more stable complex (300 times stable).
Complete step by step answer:
Haemoglobin present in red blood corpuscles (RBC) is a biochemical that plays an important role in supplying oxygen to different parts of our body.
The structure of haemoglobin comprises four heme groups and polypeptide chains. Each heme group has an iron atom which has six coordination sites. Fe (II) is coordinated to four nitrogen atoms of a porphyrin ring. The fifth coordination site is occupied by the N-atom of histidine (an amino acid) group in the polypeptide chain. The sixth site remains vacant.
Haemoglobin picks up oxygen from the lungs and carries it to the tissues via the circulatory system. The sixth vacant position is then occupied by dioxygen (${{O}_{2}}$) to form an oxyhaemoglobin complex.
\[Hb+{{O}_{2}}\rightleftarrows Hb{{O}_{2}}\]
But we know that CO is a stronger ligand than${{O}_{2}}$. So, if CO is present in the surrounding air in excess, it combines with haemoglobin by linking to the Fe (II) at the sixth coordination site. The complex thus formed is called carboxyhemoglobin and it is 300 times more stable than oxyhaemoglobin.
\[Hb+CO\rightleftarrows HbCO\]
Thus, the supply of oxygen of oxygen to different tissues is prevented due to linkage of CO with haemoglobin. When the concentration of carboxyhemoglobin reaches about 3-4% in blood, the ability of haemoglobin to bind with oxygen is largely reduced.
The deficiency of oxygen results in difficulty in breathing, headache, dizziness, in certain cases where the concentration of CO in the surrounding air is more than 750 ppm, it leads to acute oxygen starvation, i.e. anoxia and results in coma and death.

Thus, the reason for the poisonous nature of CO is its ability to form a stable complex with haemoglobin.
Additional Information:
The maximum concentration of CO in the surrounding air should be 40 ppm for an exposure of 6-8 hours. Concentration of CO in the body of smokers is more than 5% and they are highly prone to acute oxygen starvation.
CO is produced from incomplete combustion of carbon. Patients suffering from CO poisoning are kept in high pressure oxygen containing chambers. The pressure of oxygen in those chambers is about 2.5 atm and thus, CO in haemoglobin is replaced by ${{O}_{2}}$.
\[HbCO+{{O}_{2}}\rightleftarrows Hb{{O}_{2}}+CO\]
Note:Under normal conditions, ${{O}_{2}}$ binds with haemoglobin to form oxyhaemoglobin. But, if the concentration of CO is more in the surrounding air then, haemoglobin forms complexes with CO and not ${{O}_{2}}$. This is because CO combines with haemoglobin more easily (about 200 times faster) than ${{O}_{2}}$ to form a more stable complex (300 times stable).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




