
How many structural isomers with the molecular formula ${C_4}{H_{10}}O$ give infrared absorptions both at approximately 1200 $c{m^{ - 1}}$ and at approximately 3400 $c{m^{ - 1}}$?
a.) 2
b.) 4
c.) 6
d.) 7
Answer
600.3k+ views
Hint: The hydroxyl functional group (-OH) has a broad region of spectrum, generally 2500 to 4000 $c{m^{ - 1}}$. The region below 1200 $c{m^{ - 1}}$ is known as the fingerprint region. It accounts for the C-C, C-O and C-N vibrations. The region above 1200 $c{m^{ - 1}}$ is known as the group frequency region.
Complete step by step answer:
The region of the infrared spectrum from 1200 to 700 $c{m^{ - 1}}$ is called the fingerprint region. Many different vibrations, including C-O, C-C and C-N single bond stretches, C-H bending vibrations, and some bands due to benzene rings are found in this region. This region is usually the most confusing and complex region to interpret. However, the utility of the fingerprint region is that the many bands there provide a fingerprint for a molecule.
Many functional groups absorb infrared radiation at about the same wavenumber, regardless of the structure of the rest of the molecule. For example, C-H stretching vibrations usually appear between 3200 and 2800 $c{m^{ - 1}}$ and hydroxyl(-OH) stretching vibrations usually appear between 2500 and 4000 $c{m^{ - 1}}$. This makes these bands diagnostic markers for the presence of a functional group in a sample. These types of infrared bands are called group frequencies because they tell us about the presence or absence of specific functional groups in a sample.
Now, in the given question, as one IR absorption occurs at 3400 $c{m^{ - 1}}$, then one -OH group is bound to be present in the structure. Also, as only one oxygen is present in the molecular formula of the compound, the other absorption at 1200 $c{m^{ - 1}}$ is due to the C-C bond.
Hence, the total structures of ${C_4}{H_{10}}O$ having hydroxyl group are 4
n-Butanol
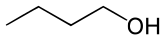
Sec-Butanol
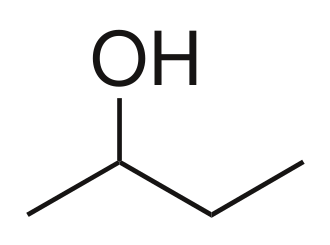
Tert-Butanol
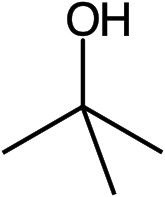
Iso-butanol
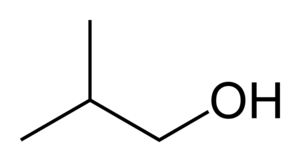
Hence, the correct answer is (B) 4.
Note: A student might confuse the absorption at 1200 $c{m^{ - 1}}$ to be due to C-O bond which is not the case. Due to the presence of a single oxygen, then the absorption at 3400 $c{m^{ - 1}}$ wouldn’t exist. When intermolecular hydrogen bonding occurs in the functional alcohol group, the IR frequency is accompanied by a shift to lower frequency.
Complete step by step answer:
The region of the infrared spectrum from 1200 to 700 $c{m^{ - 1}}$ is called the fingerprint region. Many different vibrations, including C-O, C-C and C-N single bond stretches, C-H bending vibrations, and some bands due to benzene rings are found in this region. This region is usually the most confusing and complex region to interpret. However, the utility of the fingerprint region is that the many bands there provide a fingerprint for a molecule.
Many functional groups absorb infrared radiation at about the same wavenumber, regardless of the structure of the rest of the molecule. For example, C-H stretching vibrations usually appear between 3200 and 2800 $c{m^{ - 1}}$ and hydroxyl(-OH) stretching vibrations usually appear between 2500 and 4000 $c{m^{ - 1}}$. This makes these bands diagnostic markers for the presence of a functional group in a sample. These types of infrared bands are called group frequencies because they tell us about the presence or absence of specific functional groups in a sample.
Now, in the given question, as one IR absorption occurs at 3400 $c{m^{ - 1}}$, then one -OH group is bound to be present in the structure. Also, as only one oxygen is present in the molecular formula of the compound, the other absorption at 1200 $c{m^{ - 1}}$ is due to the C-C bond.
Hence, the total structures of ${C_4}{H_{10}}O$ having hydroxyl group are 4
n-Butanol

Sec-Butanol

Tert-Butanol

Iso-butanol

Hence, the correct answer is (B) 4.
Note: A student might confuse the absorption at 1200 $c{m^{ - 1}}$ to be due to C-O bond which is not the case. Due to the presence of a single oxygen, then the absorption at 3400 $c{m^{ - 1}}$ wouldn’t exist. When intermolecular hydrogen bonding occurs in the functional alcohol group, the IR frequency is accompanied by a shift to lower frequency.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




