
How many structural isomers are possible with molecular ${C_8}{H_{10}}$?
Answer
577.5k+ views
Hint: We can define isomers as molecules that have the same molecular form but they have a difference in the arrangement of the atoms in space. The phenomenon of these isomers is called isomers.
Complete step by step answer:
We know that structural isomers of the compounds have the same molecular formula but difference in the spatial arrangement of atoms and the phenomenon is called structural isomerism. As per IUPAC naming, we can call structural isomerism as constitutional isomerism. If we increase the number of carbon atoms in the alkane molecule, there could be an increase in structural isomers.
We can classify structural isomers into three types,
1) Chain isomers: The carbon atoms are arranged in various orders.
2) Position isomers: The carbon skeleton remains unchanged, but the position of the functional group is varied. This kind of isomer is called position isomer and the isomerism is called positional isomerism.
Example: Compounds with molecular formula ${C_3}{H_7}Br$ will be 1-bromopropane and 2-bromopropane. The structures are drawn as,
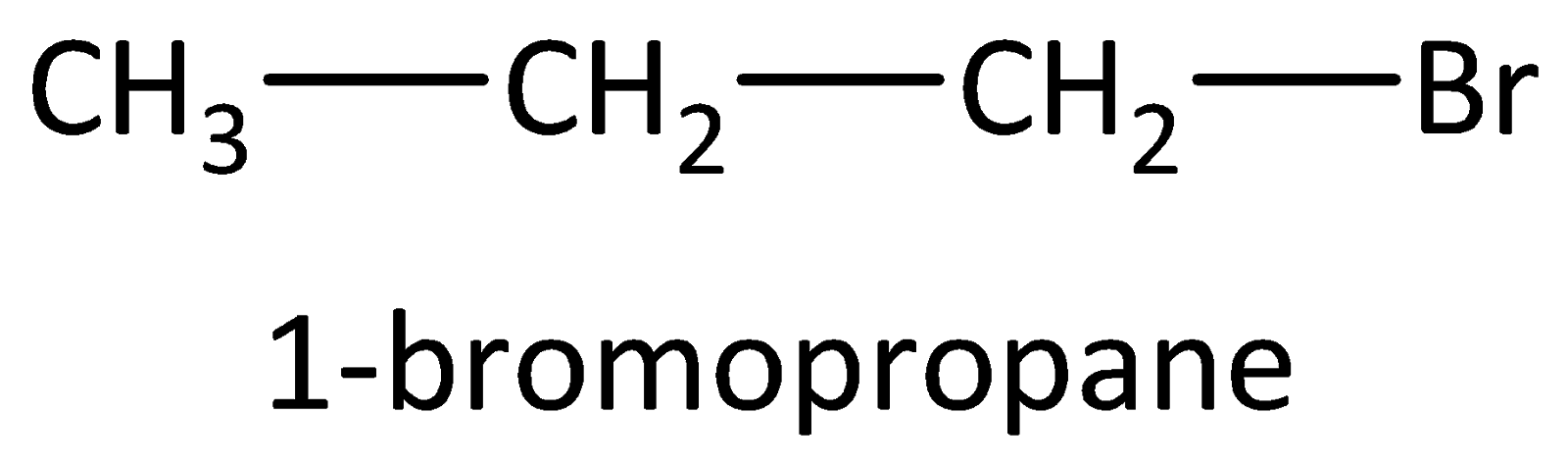
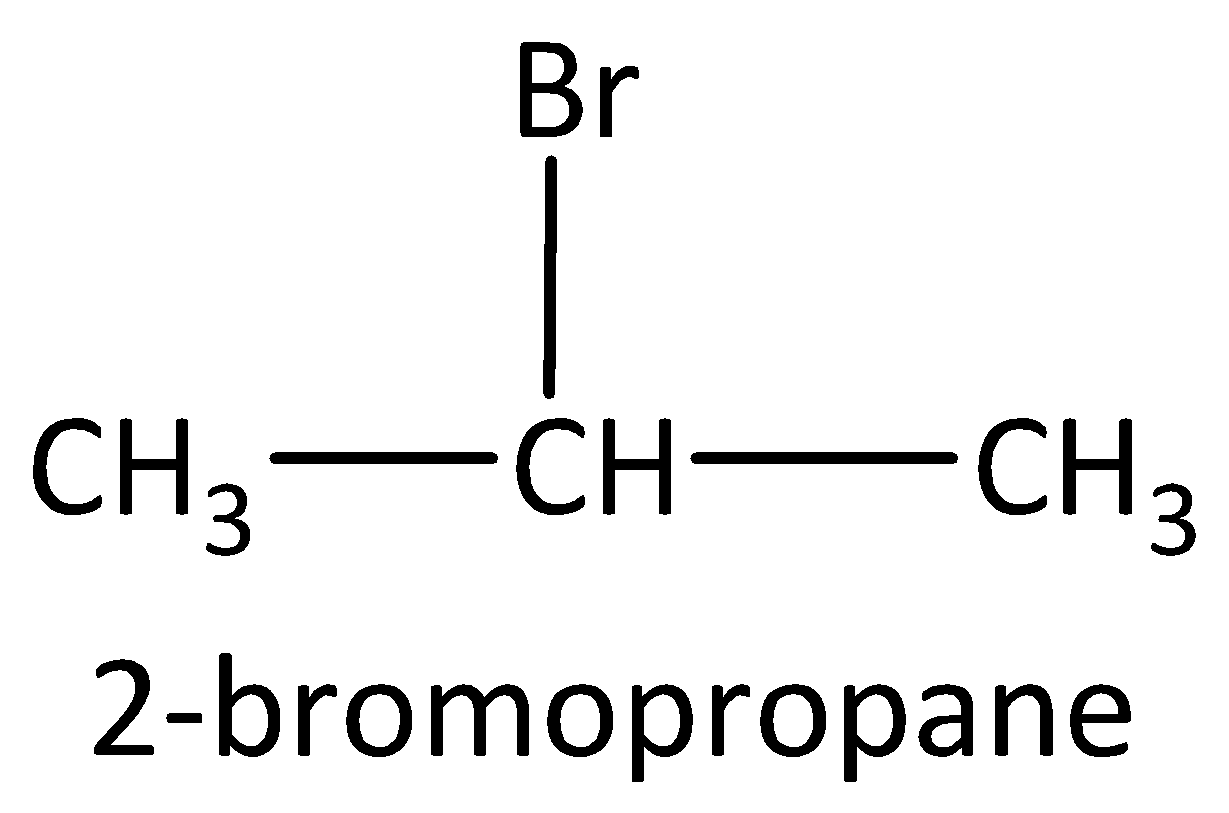
3) Functional group isomers: The arrangement of atoms to make various different functional groups is called as functional group isomers and the isomerism is called as functional group isomerism.
Example: Compounds with molecular formula ${C_3}{H_6}O$ will be either propanal (aldehyde) or a propanone (ketone). The structures are drawn as,
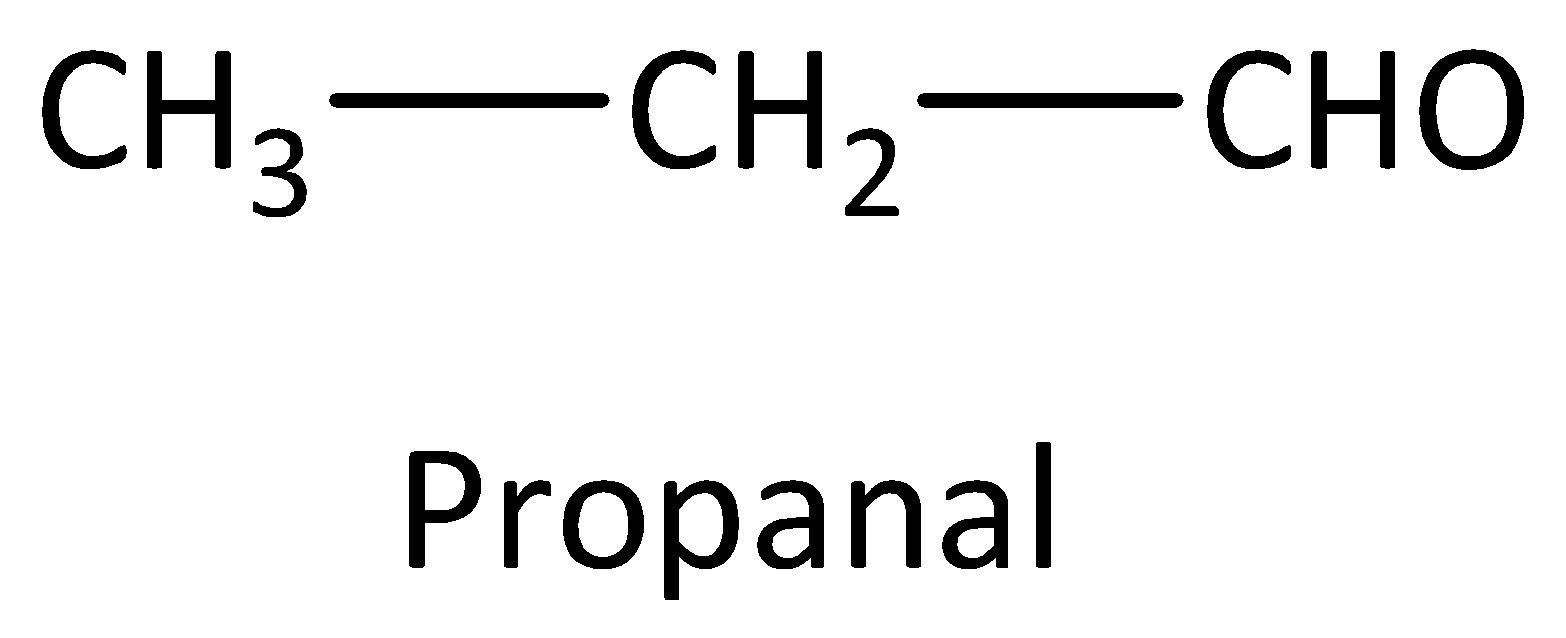
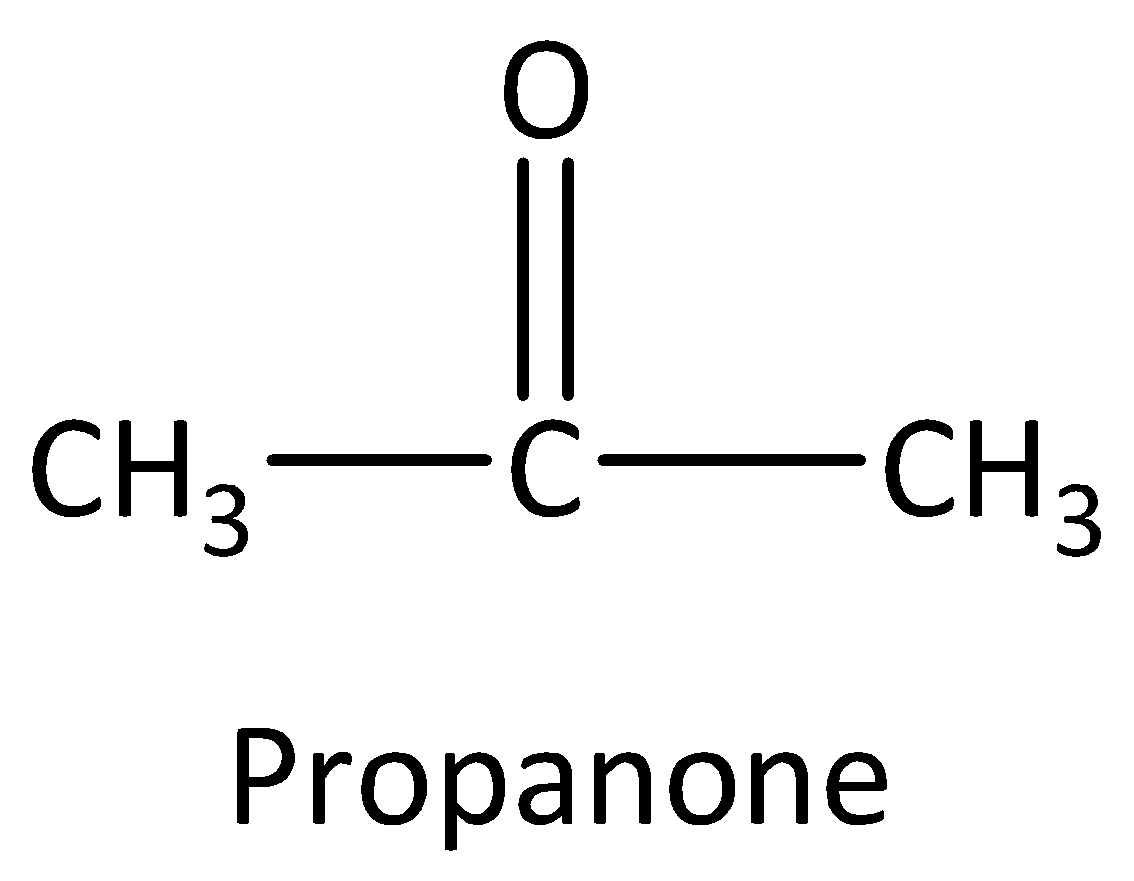
We can calculate the number of structural isomers using the formula,
Double bond equivalent=$C + 1 - \dfrac{H}{2}$
Here C is the number of atoms in carbon and H is the number of atoms in hydrogen.
In ${C_8}{H_{10}}$, the number of carbon atoms is eight and the number of hydrogen atoms is ten.
We can substitute the values of carbon atoms and hydrogen in the formula,
Double bond equivalent=$8 + 1 - \dfrac{{10}}{2}$
Double bond equivalent=$4$
Since the double bond equivalent is 4. There would be either 4 double bond (or) 1 ring with 3-double bond (or) 2-double bond, 2 ring (or) 2 triple bond etc.
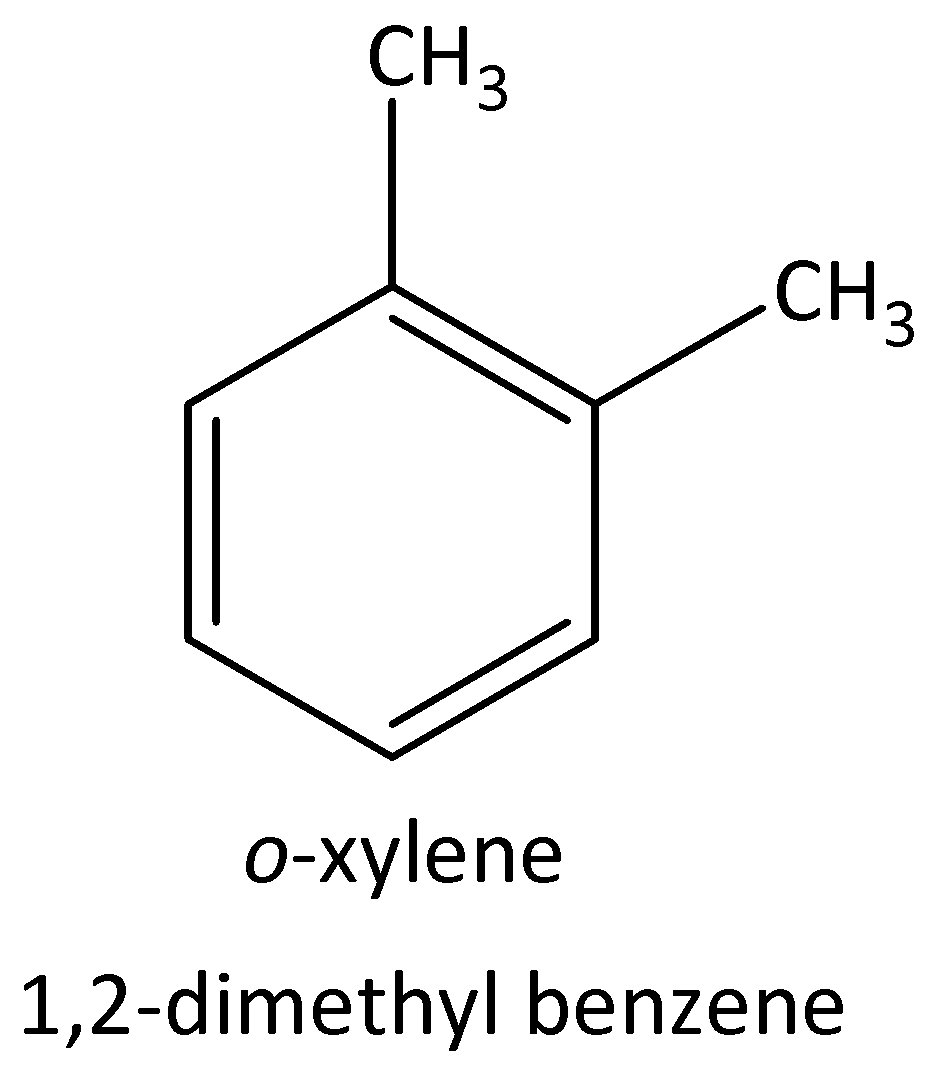
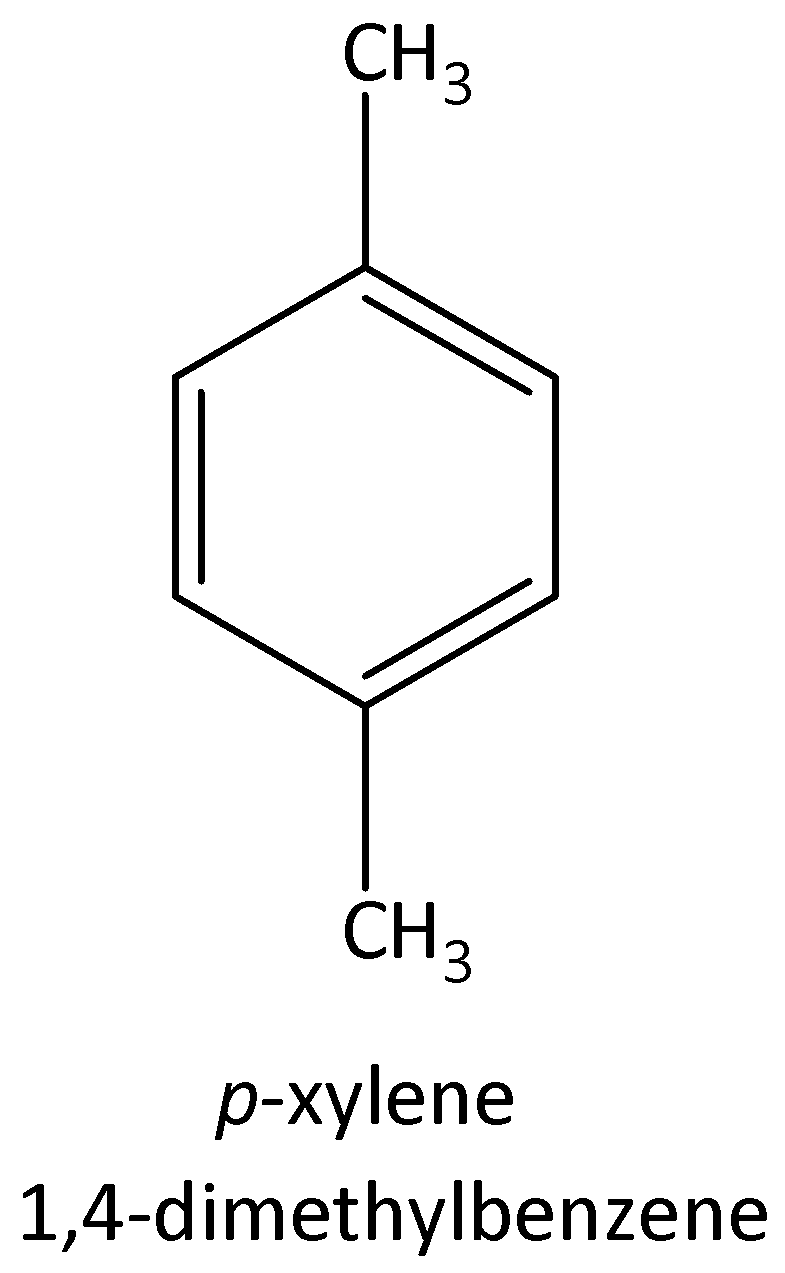
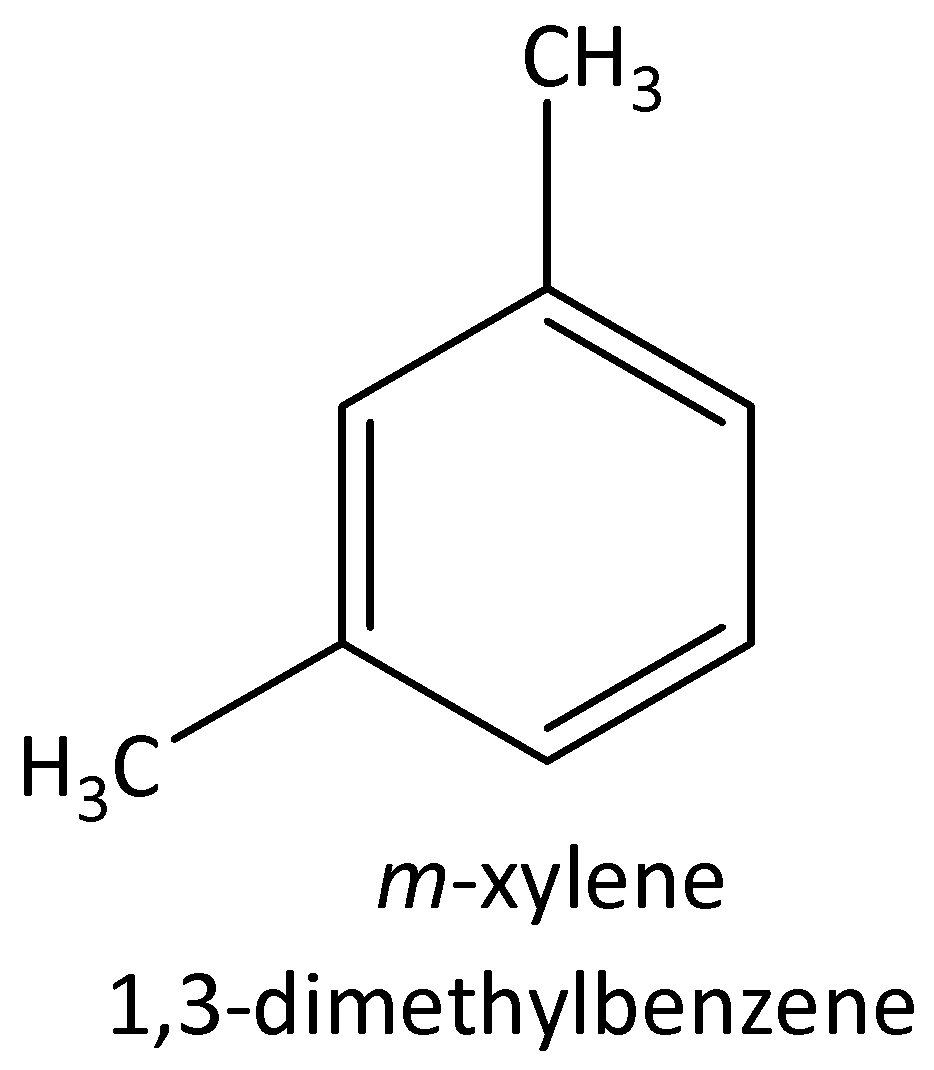
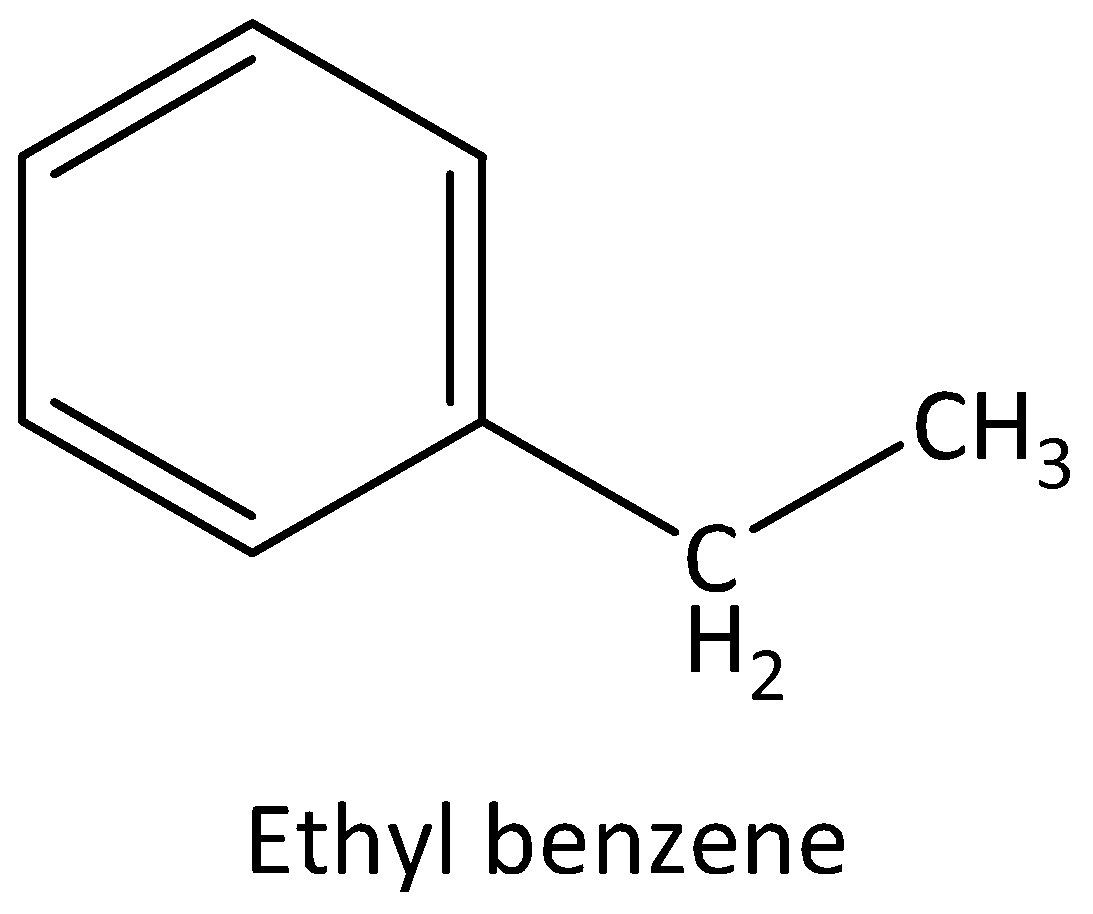
The four possible structural isomers of ${C_8}{H_{10}}$ are,
$1,2 - $dimethylbenzene
$1,3 - $dimethylbenzene
$1,4 - $dimethylbenzene
Ethylbenzene
Note:
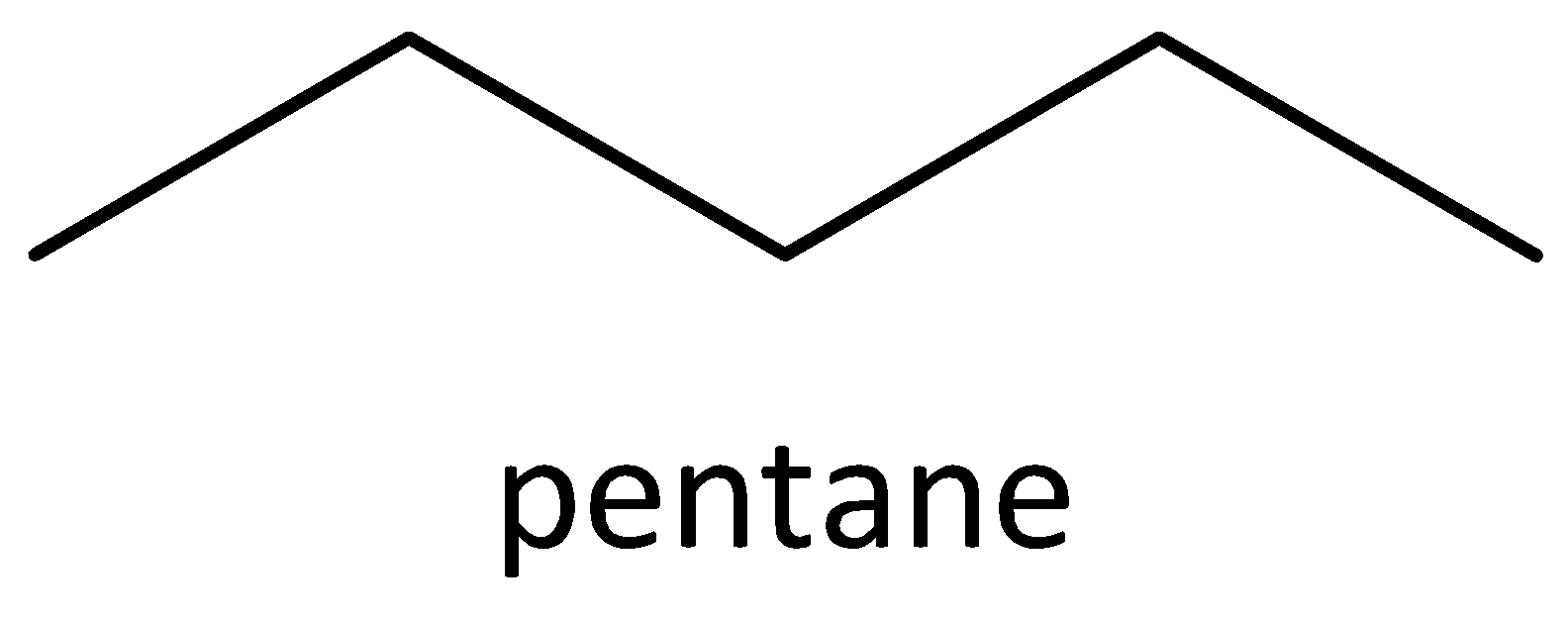
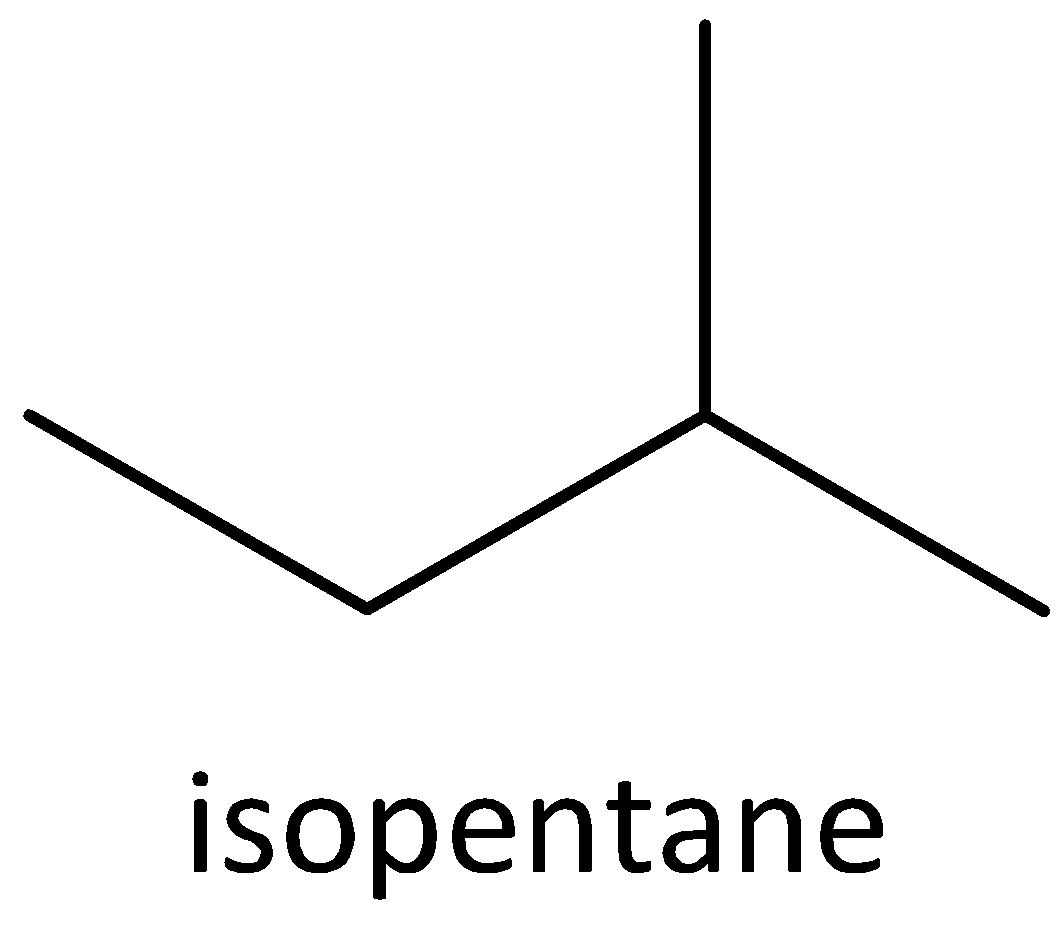
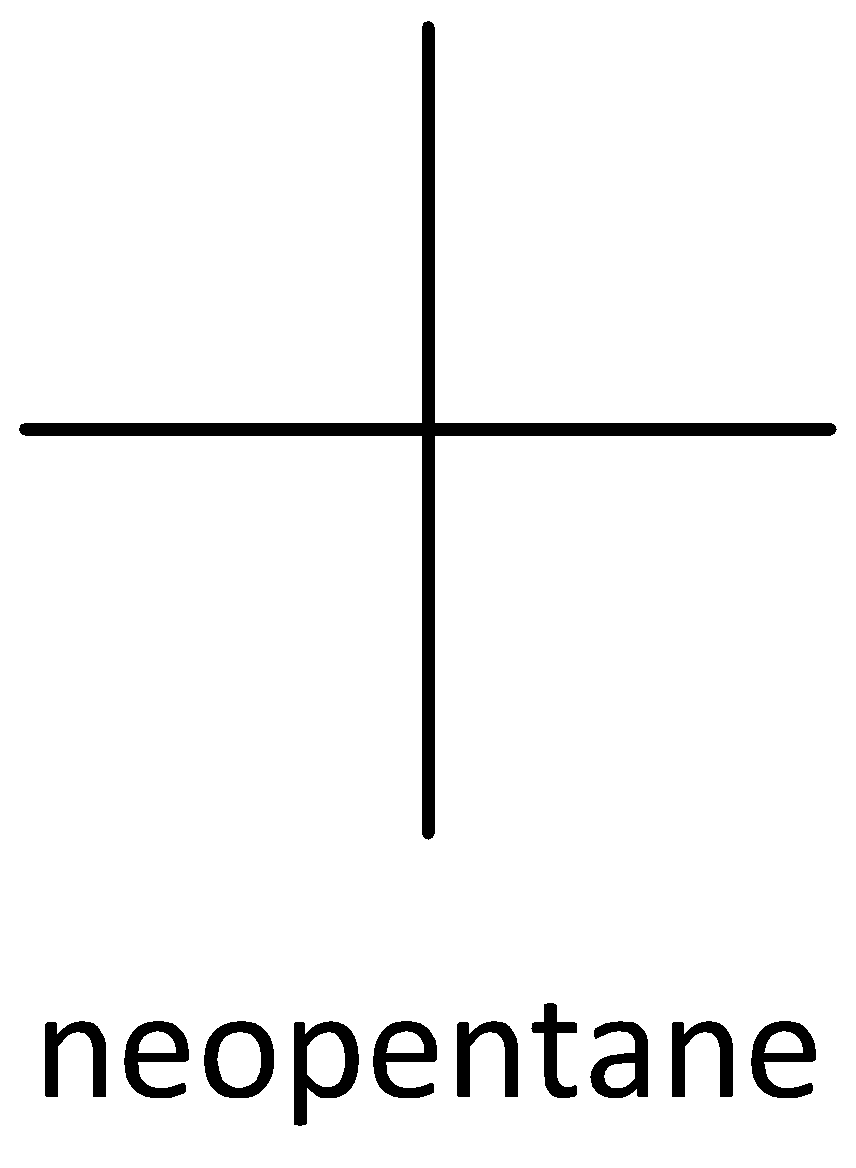
We must know that the structural isomers have different chemical properties such as lower melting and boiling points etc. Structural isomers are opposite of stereoisomers. Examples of stereoisomers are cis 2-butene and trans 2-butene. An example of chain isomer is n-pentane, isopentane and neopentane.
Complete step by step answer:
We know that structural isomers of the compounds have the same molecular formula but difference in the spatial arrangement of atoms and the phenomenon is called structural isomerism. As per IUPAC naming, we can call structural isomerism as constitutional isomerism. If we increase the number of carbon atoms in the alkane molecule, there could be an increase in structural isomers.
We can classify structural isomers into three types,
1) Chain isomers: The carbon atoms are arranged in various orders.
2) Position isomers: The carbon skeleton remains unchanged, but the position of the functional group is varied. This kind of isomer is called position isomer and the isomerism is called positional isomerism.
Example: Compounds with molecular formula ${C_3}{H_7}Br$ will be 1-bromopropane and 2-bromopropane. The structures are drawn as,
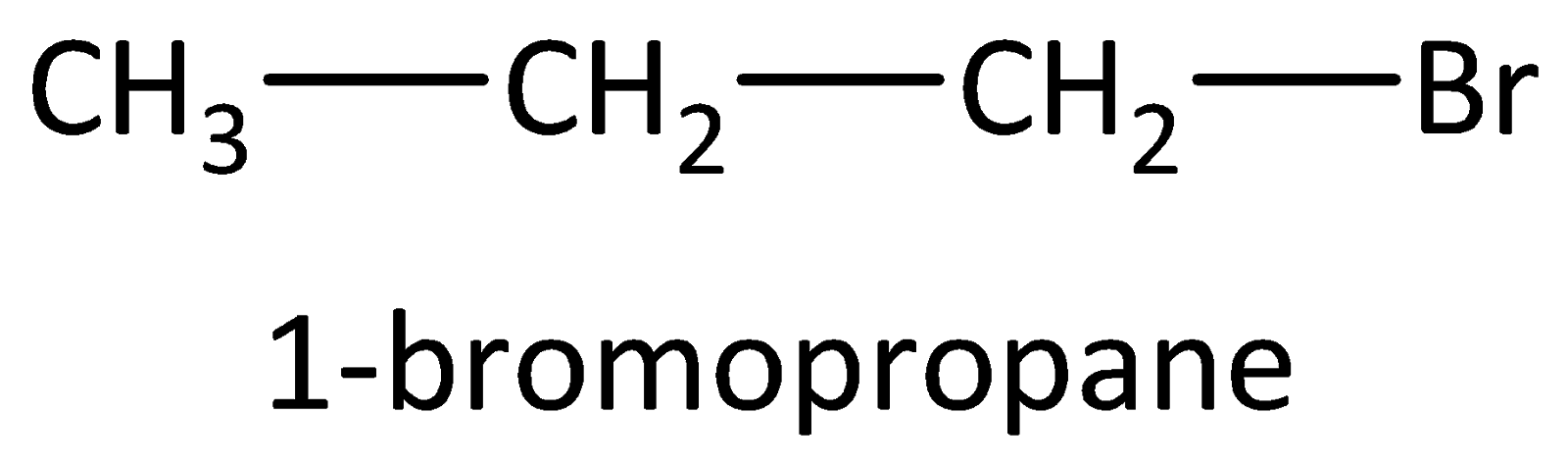

3) Functional group isomers: The arrangement of atoms to make various different functional groups is called as functional group isomers and the isomerism is called as functional group isomerism.
Example: Compounds with molecular formula ${C_3}{H_6}O$ will be either propanal (aldehyde) or a propanone (ketone). The structures are drawn as,


We can calculate the number of structural isomers using the formula,
Double bond equivalent=$C + 1 - \dfrac{H}{2}$
Here C is the number of atoms in carbon and H is the number of atoms in hydrogen.
In ${C_8}{H_{10}}$, the number of carbon atoms is eight and the number of hydrogen atoms is ten.
We can substitute the values of carbon atoms and hydrogen in the formula,
Double bond equivalent=$8 + 1 - \dfrac{{10}}{2}$
Double bond equivalent=$4$
Since the double bond equivalent is 4. There would be either 4 double bond (or) 1 ring with 3-double bond (or) 2-double bond, 2 ring (or) 2 triple bond etc.
The four possible structural isomers of ${C_8}{H_{10}}$ are,
$1,2 - $dimethylbenzene
$1,3 - $dimethylbenzene
$1,4 - $dimethylbenzene
Ethylbenzene




Note:
We must know that the structural isomers have different chemical properties such as lower melting and boiling points etc. Structural isomers are opposite of stereoisomers. Examples of stereoisomers are cis 2-butene and trans 2-butene. An example of chain isomer is n-pentane, isopentane and neopentane.



Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




