
How many single carbon – carbon bonds are present in propane?
Answer
521.1k+ views
Hint :To solve this question, we need to know and understand the structure of the propane and number of carbon and hydrogen atoms involved in it. Propane is a colourless gas and has an odour and is used as a source for energy production often, in the form of LPG (Liquefied petroleum gas) in the combination with some other gases like butane.
Complete Step By Step Answer:
So, propane consists of three carbon atoms and eight hydrogen atoms having a molecular formula - $ {C_3}{H_8} $ .
So, let’s first draw the structure for the better understanding of bonds.
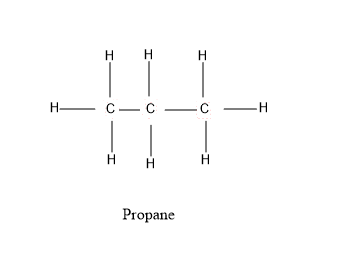
As we can see from the diagram that, there are two single carbon – carbon bonds i.e $ C - C $ and eight $ C - H $ bonds present .These bonds altogether form a stable propane molecule structure.
Therefore, the answer to our question is how many single carbon – carbon bonds are present in propane $ 2 $ . There are two single carbon – carbon bonds ( $ C - C $ ) present in the centre of the atom.
Note :
Often isomerism is important for the compound, because a slight difference in the structure results in the great difference in the physical and chemical properties of the compound. And in the case of propane, we do not have isomers for it, because of the presence of less carbon atoms. As in propane we have only three carbon atoms, and for a side chain to form we need more than three carbon atoms, hence there is no formation of side chain in propane and therefore, no isomer.
Complete Step By Step Answer:
So, propane consists of three carbon atoms and eight hydrogen atoms having a molecular formula - $ {C_3}{H_8} $ .
So, let’s first draw the structure for the better understanding of bonds.

As we can see from the diagram that, there are two single carbon – carbon bonds i.e $ C - C $ and eight $ C - H $ bonds present .These bonds altogether form a stable propane molecule structure.
Therefore, the answer to our question is how many single carbon – carbon bonds are present in propane $ 2 $ . There are two single carbon – carbon bonds ( $ C - C $ ) present in the centre of the atom.
Note :
Often isomerism is important for the compound, because a slight difference in the structure results in the great difference in the physical and chemical properties of the compound. And in the case of propane, we do not have isomers for it, because of the presence of less carbon atoms. As in propane we have only three carbon atoms, and for a side chain to form we need more than three carbon atoms, hence there is no formation of side chain in propane and therefore, no isomer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




